Computational Analysis of the Influence of Menopause and Ageing on Bone Mineral Density, Exploring the Impact of Bone Turnover and Focal Bone Balance-A Study on Overload and Underload Scenarios
- PMID: 38004295
- PMCID: PMC10672644
- DOI: 10.3390/life13112155
Computational Analysis of the Influence of Menopause and Ageing on Bone Mineral Density, Exploring the Impact of Bone Turnover and Focal Bone Balance-A Study on Overload and Underload Scenarios
Abstract
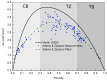
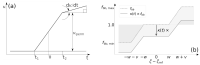
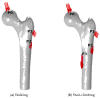
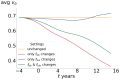
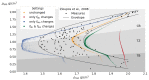
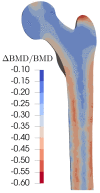
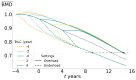
This study aims to investigate the impact of hormonal imbalances during menopause, compounded by the natural ageing process, on bone health. Specifically, it examines the effects of increased bone turnover and focal bone balance on bone mass. A three-dimensional computational bone remodeling model was employed to simulate the response of the femur to habitual loads over a 19-year period, spanning premenopause, menopause, and postmenopause. The model was calibrated using experimental bone mineral density data from the literature to ensure accurate simulations. The study reveals that individual alterations in bone turnover or focal bone balance do not fully account for the observed experimental outcomes. Instead, simultaneous changes in both factors provide a more comprehensive explanation, leading to increased porosity while maintaining the material-to-apparent density ratio. Additionally, different load scenarios were tested, demonstrating that reaching the clinical osteoporosis threshold is independent of the timing of load changes. However, underload scenarios resulted in the threshold being reached approximately 6 years earlier than overload scenarios. These findings hold significant implications for strategies aimed at delaying the onset of osteoporosis and minimizing fracture risks through targeted mechanical stimulation during the early stages of menopause.
Keywords: bone health; bone remodeling; computational simulation; mathematical model; menopause; osteoporosis.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
A theoretical analysis of the changes in basic multicellular unit activity at menopause.Bone. 2003 Apr;32(4):357-63. doi: 10.1016/s8756-3282(03)00037-1. Bone. 2003. PMID: 12689678
-
Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis.J Bone Miner Res. 1996 Mar;11(3):337-49. doi: 10.1002/jbmr.5650110307. J Bone Miner Res. 1996. PMID: 8852944
-
Balance and functional mobility predict low bone mineral density among postmenopausal women undergoing recent menopause with osteoporosis, osteopenia, and normal bone mineral density: A cross-sectional study.Geriatr Nurs. 2021 Jan-Feb;42(1):33-36. doi: 10.1016/j.gerinurse.2020.10.020. Epub 2020 Nov 19. Geriatr Nurs. 2021. PMID: 33221555
-
Physical activity in the prevention and amelioration of osteoporosis in women : interaction of mechanical, hormonal and dietary factors.Sports Med. 2005;35(9):779-830. doi: 10.2165/00007256-200535090-00004. Sports Med. 2005. PMID: 16138787 Review.
-
Bone densitometry in premenopausal women: synthesis and review.J Clin Densitom. 2004 Spring;7(1):85-92. doi: 10.1385/jcd:7:1:85. J Clin Densitom. 2004. PMID: 14742892 Review.
Cited by
-
Temporal declines in bone mineral density and trabecular bone score during androgen deprivation therapy.J Bone Miner Metab. 2024 Nov;42(6):668-674. doi: 10.1007/s00774-024-01537-z. Epub 2024 Sep 12. J Bone Miner Metab. 2024. PMID: 39266779
References
-
- Frost H.M. Dynamics of bone remodeling. Bone Biodyn. 1964;14:315–333.
-
- Ansari N., Sims N.A. Bone Regulators and Osteoporosis Therapy. Springer International Publishing; Berlin/Heidelberg, Germany: 2019. The Cells of Bone and Their Interactions; pp. 1–25. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

