On the Emergence of Autonomous Chemical Systems through Dissipation Kinetics
- PMID: 38004311
- PMCID: PMC10672272
- DOI: 10.3390/life13112171
On the Emergence of Autonomous Chemical Systems through Dissipation Kinetics
Abstract
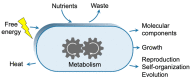
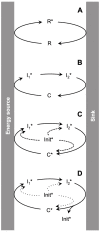
This work addresses the kinetic requirements for compensating the entropic cost of self-organization and natural selection, thereby revealing a fundamental principle in biology. Metabolic and evolutionary features of life cannot therefore be separated from an origin of life perspective. Growth, self-organization, evolution and dissipation processes need to be metabolically coupled and fueled by low-entropy energy harvested from the environment. The evolutionary process requires a reproduction cycle involving out-of-equilibrium intermediates and kinetic barriers that prevent the reproductive cycle from proceeding in reverse. Model analysis leads to the unexpectedly simple relationship that the system should be fed energy with a potential exceeding a value related to the ratio of the generation time to the transition state lifetime, thereby enabling a process mimicking natural selection to take place. Reproducing life's main features, in particular its Darwinian behavior, therefore requires satisfying constraints that relate to time and energy. Irreversible reaction cycles made only of unstable entities reproduce some of these essential features, thereby offering a physical/chemical basis for the possible emergence of autonomy. Such Emerging Autonomous Systems (EASs) are found to be capable of maintaining and reproducing their kind through the transmission of a stable kinetic state, thereby offering a physical/chemical basis for what could be deemed an epigenetic process.
Keywords: autocatalysis; dynamic kinetic stability; emerging autonomous systems; evolution; irreversibility; origin of life.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
On the conditions for mimicking natural selection in chemical systems.Nat Rev Chem. 2020 Feb;4(2):102-109. doi: 10.1038/s41570-019-0155-6. Epub 2020 Jan 14. Nat Rev Chem. 2020. PMID: 37128049 Review.
-
Towards an evolutionary theory of the origin of life based on kinetics and thermodynamics.Open Biol. 2013 Nov 6;3(11):130156. doi: 10.1098/rsob.130156. Open Biol. 2013. PMID: 24196781 Free PMC article. Review.
-
Investigating the Evolution and Development of Biological Systems from the Perspective of Thermo-Kinetics and Systems Theory.Orig Life Evol Biosph. 2020 Dec;50(3-4):121-143. doi: 10.1007/s11084-020-09601-0. Epub 2020 Dec 3. Orig Life Evol Biosph. 2020. PMID: 33269436 Review.
-
The driving force for life's emergence: kinetic and thermodynamic considerations.J Theor Biol. 2003 Feb 7;220(3):393-406. doi: 10.1006/jtbi.2003.3178. J Theor Biol. 2003. PMID: 12468287
-
How and why kinetics, thermodynamics, and chemistry induce the logic of biological evolution.Beilstein J Org Chem. 2017 Apr 7;13:665-674. doi: 10.3762/bjoc.13.66. eCollection 2017. Beilstein J Org Chem. 2017. PMID: 28487761 Free PMC article.
Cited by
-
From the RNA-Peptide World: Prebiotic Reaction Conditions Compatible with Lipid Membranes for the Formation of Lipophilic Random Peptides in the Presence of Short Oligonucleotides, and More.Life (Basel). 2024 Jan 9;14(1):108. doi: 10.3390/life14010108. Life (Basel). 2024. PMID: 38255723 Free PMC article.
-
Opinion: The Key Steps in the Origin of Life to the Formation of the Eukaryotic Cell.Life (Basel). 2024 Feb 5;14(2):226. doi: 10.3390/life14020226. Life (Basel). 2024. PMID: 38398735 Free PMC article. Review.
References
-
- Sutherland J. Opinion: Studies on the origin of life—The end of the beginning. Nat. Rev. Chem. 2017;1:12. doi: 10.1038/s41570-016-0012. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

