DP2, a Carbohydrate Derivative, Enhances In Vitro Osteoblast Mineralisation
- PMID: 38004380
- PMCID: PMC10674337
- DOI: 10.3390/ph16111512
DP2, a Carbohydrate Derivative, Enhances In Vitro Osteoblast Mineralisation
Abstract
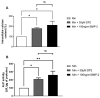
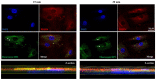
Bone fracture healing is a complex biological process involving four phases coordinated over time: hematoma formation, granulation tissue formation, bony callus formation, and bone remodelling. Bone fractures represent a significant health problem, particularly among the elderly population and patients with comorbidities. Therapeutic strategies proposed to treat such fractures include the use of autografts, allografts, and tissue engineering strategies. It has been shown that bone morphogenetic protein 2 (BMP-2) has a therapeutic potential to enhance fracture healing. Despite the clinical efficacy of BMP-2 in osteoinduction and bone repair, adverse side effects and complications have been reported. Therefore, in this in vitro study, we propose the use of a disaccharide compound (DP2) to improve the mineralisation process. We first evaluated the effect of DP2 on primary human osteoblasts (HOb), and then investigated the mechanisms involved. Our findings showed that (i) DP2 improved osteoblast differentiation by inducing alkaline phosphatase activity, osteopontin, and osteocalcin expression; (ii) DP2 induced earlier in vitro mineralisation in HOb cells compared to BMP-2 mainly by earlier activation of Runx2; and (iii) DP2 is internalized in HOb cells and activates the protein kinase C signalling pathway. Consequently, DP2 is a potential therapeutical candidate molecule for bone fracture repair.
Keywords: DP2; bone morphogenetic proteins; bone regeneration; osteoblast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




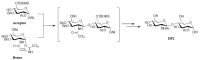

Similar articles
-
Enhancement of In Vivo Bone Regeneration by the Carbohydrate Derivative DP2.Pharmaceuticals (Basel). 2025 Feb 5;18(2):215. doi: 10.3390/ph18020215. Pharmaceuticals (Basel). 2025. PMID: 40006029 Free PMC article.
-
Alphavbeta integrins play an essential role in BMP-2 induction of osteoblast differentiation.J Bone Miner Res. 2005 Feb;20(2):330-40. doi: 10.1359/JBMR.041013. Epub 2004 Oct 18. J Bone Miner Res. 2005. PMID: 15647827
-
Harmine promotes osteoblast differentiation through bone morphogenetic protein signaling.Biochem Biophys Res Commun. 2011 Jun 3;409(2):260-5. doi: 10.1016/j.bbrc.2011.05.001. Epub 2011 May 6. Biochem Biophys Res Commun. 2011. PMID: 21570953
-
EP1(-/-) mice have enhanced osteoblast differentiation and accelerated fracture repair.J Bone Miner Res. 2011 Apr;26(4):792-802. doi: 10.1002/jbmr.272. J Bone Miner Res. 2011. PMID: 20939055 Free PMC article.
-
LMP-1 retroviral gene therapy influences osteoblast differentiation and fracture repair: a preliminary study.Calcif Tissue Int. 2008 Sep;83(3):202-11. doi: 10.1007/s00223-008-9163-0. Epub 2008 Aug 16. Calcif Tissue Int. 2008. PMID: 18709396
Cited by
-
Enhancement of In Vivo Bone Regeneration by the Carbohydrate Derivative DP2.Pharmaceuticals (Basel). 2025 Feb 5;18(2):215. doi: 10.3390/ph18020215. Pharmaceuticals (Basel). 2025. PMID: 40006029 Free PMC article.
References
-
- Willers C., Norton N., Harvey N.C., Jacobson T., Johansson H., Lorentzon M., McCloskey E.V., Borgström F., Kanis J.A. The SCOPE review panel of the IOF Osteoporosis in Europe: A Compendium of Country-Specific Reports. Arch. Osteoporos. 2022;17:23. doi: 10.1007/s11657-021-00969-8. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

