Preliminary Assessment of Polysaccharide-Based Emulgels Containing Delta-Aminolevulinic Acid for Oral Lichen planus Treatment
- PMID: 38004400
- PMCID: PMC10674658
- DOI: 10.3390/ph16111534
Preliminary Assessment of Polysaccharide-Based Emulgels Containing Delta-Aminolevulinic Acid for Oral Lichen planus Treatment
Abstract
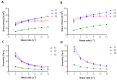
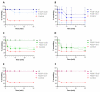
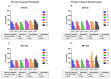
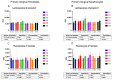
Photodynamic therapy using delta-aminolevulinic acid is considered a promising option in the treatment of oral lichen planus. In the present work, three emulgel compositions prepared from natural polysaccharide gums, tragacanth, xanthan and gellan, were preliminarily tested for oromucosal delivery of delta-aminolevulinic acid. Apart from cytotoxicity studies in two gingival cell lines, the precise goal was to investigate whether the presence of the drug altered the rheological and mucoadhesive behavior of applied gelling agents and to examine how dilution with saliva fluid influenced the retention of the designed emulgels by oromucosal tissue. Ex vivo mucoadhesive studies revealed that a combination of xanthan and gellan gum enhanced carrier retention by buccal tissue even upon dilution with the saliva. In turn, the incorporation of delta-aminolevulinic acid favored interactions with mucosal tissue, particularly formulations comprised of tragacanth. The designed preparations had no significant impact on the cell viability after a 24 h incubation in the tested concentration range. Cytotoxicity studies demonstrated that tragacanth-based and gellan/xanthan-based emulgels might exert a protective effect on the metabolic activity of human gingival fibroblasts and keratinocytes. Overall, the presented data show the potential of designed emulgels as oromucosal platforms for delta-aminolevulinic acid delivery.
Keywords: delta-aminolevulinic acid; emulgel; gellan gum; oral lichen planus; oromucosal delivery; tragacanth; xanthan.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Evaluation of Oromucosal Natural Gum-Based Emulgels as Novel Strategy for Photodynamic Therapy of Oral Premalignant Lesions.Pharmaceutics. 2023 Oct 23;15(10):2512. doi: 10.3390/pharmaceutics15102512. Pharmaceutics. 2023. PMID: 37896272 Free PMC article.
-
Evaluation of the impact of tragacanth/xanthan gum interpolymer complexation with chitosan on pharmaceutical performance of gels with secnidazole as potential periodontal treatment.Eur J Pharm Sci. 2024 Jan 1;192:106657. doi: 10.1016/j.ejps.2023.106657. Epub 2023 Nov 30. Eur J Pharm Sci. 2024. PMID: 38040098
-
Synergistic interactions between konjac-mannan and xanthan/tragacanth gums in tomato ketchup: Physical, rheological, and textural properties.J Texture Stud. 2018 Dec;49(6):586-594. doi: 10.1111/jtxs.12359. Epub 2018 Oct 1. J Texture Stud. 2018. PMID: 30187474
-
delta-Aminolevulinic acid and blue light photodynamic therapy for treatment of multiple basal cell carcinomas in two patients with nevoid basal cell carcinoma syndrome.Dermatol Surg. 2004 Jul;30(7):1054-61. doi: 10.1111/j.1524-4725.2004.30317.x. Dermatol Surg. 2004. PMID: 15209801 Review.
-
A review on tragacanth gum: A promising natural polysaccharide in drug delivery and cell therapy.Int J Biol Macromol. 2023 Jun 30;241:124343. doi: 10.1016/j.ijbiomac.2023.124343. Epub 2023 Apr 11. Int J Biol Macromol. 2023. PMID: 37054856 Review.
References
-
- Sulewska M., Duraj E., Sobaniec S., Graczyk A., Milewski R., Wróblewska M., Pietruski J., Pietruska M. A Clinical Evaluation of the Efficacy of Photodynamic Therapy in the Treatment of Erosive Oral Lichen Planus: A Case Series. Photodiagn. Photodyn. Ther. 2017;18:12–19. doi: 10.1016/j.pdpdt.2017.01.178. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

