GluN2A and GluN2B N-Methyl-D-Aspartate Receptor (NMDARs) Subunits: Their Roles and Therapeutic Antagonists in Neurological Diseases
- PMID: 38004401
- PMCID: PMC10674917
- DOI: 10.3390/ph16111535
GluN2A and GluN2B N-Methyl-D-Aspartate Receptor (NMDARs) Subunits: Their Roles and Therapeutic Antagonists in Neurological Diseases
Abstract
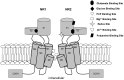
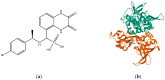
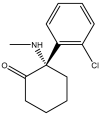
N-methyl-D-aspartate receptors (NMDARs) are ion channels that respond to the neurotransmitter glutamate, playing a crucial role in the permeability of calcium ions and excitatory neurotransmission in the central nervous system (CNS). Composed of various subunits, NMDARs are predominantly formed by two obligatory GluN1 subunits (with eight splice variants) along with regulatory subunits GluN2 (GluN2A-2D) and GluN3 (GluN3A-B). They are widely distributed throughout the CNS and are involved in essential functions such as synaptic transmission, learning, memory, plasticity, and excitotoxicity. The presence of GluN2A and GluN2B subunits is particularly important for cognitive processes and has been strongly implicated in neurodegenerative diseases like Parkinson's disease and Alzheimer's disease. Understanding the roles of GluN2A and GluN2B NMDARs in neuropathologies provides valuable insights into the underlying causes and complexities of major nervous system disorders. This knowledge is vital for the development of selective antagonists targeting GluN2A and GluN2B subunits using pharmacological and molecular methods. Such antagonists represent a promising class of NMDA receptor inhibitors that have the potential to be developed into neuroprotective drugs with optimal therapeutic profiles.
Keywords: GluN2A and GluN2B; N-methyl-D-aspartate receptors (NMDARs); antagonists; compounds; neurodegeneration; neuroprotection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
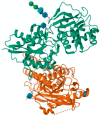
Similar articles
-
Targeting N-Methyl-d-Aspartate Receptors in Neurodegenerative Diseases.Int J Mol Sci. 2024 Mar 27;25(7):3733. doi: 10.3390/ijms25073733. Int J Mol Sci. 2024. PMID: 38612544 Free PMC article. Review.
-
Opportunities for Precision Treatment of GRIN2A and GRIN2B Gain-of-Function Variants in Triheteromeric N-Methyl-D-Aspartate Receptors.J Pharmacol Exp Ther. 2022 Apr;381(1):54-66. doi: 10.1124/jpet.121.001000. Epub 2022 Feb 2. J Pharmacol Exp Ther. 2022. PMID: 35110392 Free PMC article.
-
Synaptic NMDA receptors in basolateral amygdala principal neurons are triheteromeric proteins: physiological role of GluN2B subunits.J Neurophysiol. 2013 Mar;109(5):1391-402. doi: 10.1152/jn.00176.2012. Epub 2012 Dec 5. J Neurophysiol. 2013. PMID: 23221411
-
Alterations of NMDAR Subunits in the Cerebrospinal Fluid Across Neurodegenerative and Immunological Disorders.J Neurochem. 2025 Aug;169(8):e70192. doi: 10.1111/jnc.70192. J Neurochem. 2025. PMID: 40823742 Free PMC article.
-
Role of nonsynaptic GluN2B-containing NMDA receptors in excitotoxicity: evidence that fluoxetine selectively inhibits these receptors and may have neuroprotective effects.Brain Res Bull. 2013 Apr;93:32-8. doi: 10.1016/j.brainresbull.2012.10.005. Epub 2012 Oct 23. Brain Res Bull. 2013. PMID: 23089362 Review.
Cited by
-
Advances in the Treatment of Cognitive Impairment in Schizophrenia: Targeting NMDA Receptor Pathways.Int J Mol Sci. 2024 Oct 3;25(19):10668. doi: 10.3390/ijms251910668. Int J Mol Sci. 2024. PMID: 39408997 Free PMC article. Review.
-
The expression of insulin signaling and N-methyl-D-aspartate receptor genes in areas of gray matter atrophy is associated with cognitive function in type 2 diabetes.medRxiv [Preprint]. 2025 Apr 1:2025.03.26.25324696. doi: 10.1101/2025.03.26.25324696. medRxiv. 2025. Update in: J Alzheimers Dis. 2025 Aug 13:13872877251364906. doi: 10.1177/13872877251364906. PMID: 40236395 Free PMC article. Updated. Preprint.
-
On the functions of astrocyte-mediated neuronal slow inward currents.Neural Regen Res. 2024 Dec 1;19(12):2602-2612. doi: 10.4103/NRR.NRR-D-23-01723. Epub 2024 Mar 1. Neural Regen Res. 2024. PMID: 38595279 Free PMC article.
-
NMDA Receptors in Neurodevelopmental Disorders: Pathophysiology and Disease Models.Int J Mol Sci. 2024 Nov 18;25(22):12366. doi: 10.3390/ijms252212366. Int J Mol Sci. 2024. PMID: 39596430 Free PMC article. Review.
-
The Role of Photobiomodulation to Modulate Ion Channels in the Nervous System: A Systematic Review.Cell Mol Neurobiol. 2024 Nov 23;44(1):79. doi: 10.1007/s10571-024-01513-1. Cell Mol Neurobiol. 2024. PMID: 39579175 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

