Recent Progress in Synthesis, POM Analyses and SAR of Coumarin-Hybrids as Potential Anti-HIV Agents-A Mini Review
- PMID: 38004404
- PMCID: PMC10675815
- DOI: 10.3390/ph16111538
Recent Progress in Synthesis, POM Analyses and SAR of Coumarin-Hybrids as Potential Anti-HIV Agents-A Mini Review
Abstract
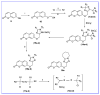
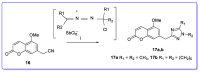
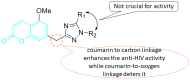
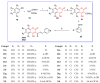
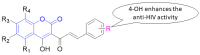
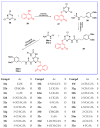
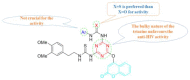
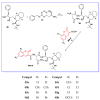
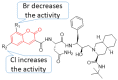
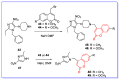
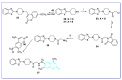
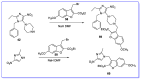
The human immunodeficiency virus (HIV) is the primary cause of acquired immune deficiency syndrome (AIDS), one of the deadliest pandemic diseases. Various mechanisms and procedures have been pursued to synthesise several anti-HIV agents, but due to the severe side effects and multidrug resistance spawning from the treatment of HIV/AIDS using highly active retroviral therapy (HAART), it has become imperative to design and synthesise novel anti-HIV agents. Literature has shown that natural sources, particularly the plant kingdom, can release important metabolites that have several biological, mechanistic and structural representations similar to chemically synthesised compounds. Certainly, compounds from natural and ethnomedicinal sources have proven to be effective in the management of HIV/AIDS with low toxicity, fewer side effects and affordability. From plants, fungi and bacteria, coumarin can be obtained, which is a secondary metabolite and is well known for its actions in different stages of the HIV replication cycle: protease, integrase and reverse transcriptase (RT) inhibition, cell membrane fusion and viral host attachment. These, among other reasons, are why coumarin moieties will be the basis of a good building block for the development of potent anti-HIV agents. This review aims to outline the synthetic pathways, structure-activity relationship (SAR) and POM analyses of coumarin hybrids with anti-HIV activity, detailing articles published between 2000 and 2023.
Keywords: AIDS; HAART; HIV; POM; coumarin; reverse transcriptase; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Coumarin-based derivatives with potential anti-HIV activity.Fitoterapia. 2021 Apr;150:104863. doi: 10.1016/j.fitote.2021.104863. Epub 2021 Feb 11. Fitoterapia. 2021. PMID: 33582266 Review.
-
Natural products and synthetic analogues against HIV: A perspective to develop new potential anti-HIV drugs.Eur J Med Chem. 2022 Apr 5;233:114217. doi: 10.1016/j.ejmech.2022.114217. Epub 2022 Mar 2. Eur J Med Chem. 2022. PMID: 35276425 Review.
-
Therapeutic Potential of Indole Derivatives as Anti-HIV Agents: A Mini-review.Curr Top Med Chem. 2022;22(12):993-1008. doi: 10.2174/1568026621666211012111901. Curr Top Med Chem. 2022. PMID: 34636313 Review.
-
Human immunodeficiency virus and host cell lipids. Interesting pathways in research for a new HIV therapy.Prog Lipid Res. 2002 Jan;41(1):27-65. doi: 10.1016/s0163-7827(01)00019-4. Prog Lipid Res. 2002. PMID: 11694268 Review.
-
Coumarins as inhibitors of HIV reverse transcriptase.Curr HIV Res. 2006 Jul;4(3):347-63. doi: 10.2174/157016206777709393. Curr HIV Res. 2006. PMID: 16842086 Review.
Cited by
-
A facile and green procedure in preparing dibenzo-chromeno-phenazine-diones using an effectual and recyclable Brønsted acidic ionic liquid.Sci Rep. 2024 Nov 5;14(1):26758. doi: 10.1038/s41598-024-73257-3. Sci Rep. 2024. PMID: 39500919 Free PMC article.
-
Design of Novel Imidazole Derivatives as Potential Non-nucleoside Reverse Transcriptase Inhibitors using Molecular Docking and Dynamics Strategies.Curr Pharm Des. 2025;31(1):65-82. doi: 10.2174/0113816128322984240725055333. Curr Pharm Des. 2025. PMID: 39354774
-
Elucidating the Mechanism of Coumarin Homodimerization Using 3-Acetylcoumarin Derivatives.Molecules. 2025 Feb 1;30(3):651. doi: 10.3390/molecules30030651. Molecules. 2025. PMID: 39942755 Free PMC article.
References
-
- Kumar V., Joshi H.C., Pandey I.P. Reverse Transcriptase Inhibition: A Way to Defeat HIV. HIV AIDS Rev. 2022;21:3–9. doi: 10.5114/hivar.2022.112762. - DOI
-
- Tariq M., Sirajuddin M., Ali S., Khalid N., Tahir M.N., Khan H., Ansari T.M. Pharmacological Investigations and Petra/Osiris/Molinspiration (POM) Analyses of Newly Synthesized Potentially Bioactive Organotin(IV) Carboxylates. J. Photochem. Photobiol. B. 2016;158:174–183. doi: 10.1016/j.jphotobiol.2016.02.028. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

