IL12/23 Blockade with Ustekinumab as a Treatment for Immune-Related Cutaneous Adverse Events
- PMID: 38004414
- PMCID: PMC10674871
- DOI: 10.3390/ph16111548
IL12/23 Blockade with Ustekinumab as a Treatment for Immune-Related Cutaneous Adverse Events
Abstract
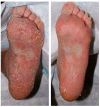
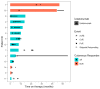
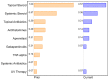
Background: Immune-related cutaneous adverse events (ircAEs) are frequent and may reduce quality of life and consistent dosing. IL12/23 has been implicated in psoriasis, which is reminiscent of the psoriasiform/lichenoid ircAE phenotype. We report the use of ustekinumab as a therapeutic option. Methods: Patients at Memorial Sloan Kettering Cancer Center, New York, who received immune checkpoint inhibitors and were treated with ustekinumab or had the keywords "ustekinumab" or "Stelara" in their clinical notes between 1 March 2017 and 1 December 2022 were retrospectively identified via a database query. Documentation from initial and follow-up visits was manually reviewed, and response to ustekinumab was categorized into complete cutaneous response (CcR, decrease to CTCAE grade 0), partial cutaneous response (PcR, any decrease in CTCAE grade exclusive of decrease to grade 0), and no cutaneous response (NcR, no change in CTCAE grade or worsening). Labs including complete blood count (CBC), cytokine panels, and IgE were obtained in a subset of patients as standard of care. Skin biopsies were reviewed by a dermatopathologist. Results: Fourteen patients with psoriasiform (85.7%), maculopapular (7.1%), and pyoderma gangrenosum (7.1%) ircAEs were identified. Ten (71.4%) receiving ustekinumab had a positive response to treatment. Among these 10 responders, 4 (40%) demonstrated partial cutaneous response and 6 (60%) demonstrated complete cutaneous resolution. Six patients (42.9%) experienced interruptions to their checkpoint inhibitor treatment as a result of intolerable ircAEs, and following ircAE management with ustekinumab, two (33.3%) were successfully rechallenged with their checkpoint inhibitors. On histopathology, patients primarily had findings of interface or psoriasiform dermatitis. No patients reported an adverse event related to ustekinumab. Conclusions: Ustekinumab showed a benefit in a subset of patients with psoriasiform/lichenoid ircAEs. No safety signals were identified. However, further prospective randomized controlled trials are needed to confirm our findings.
Keywords: biologics; dermatologic adverse events; immune-related adverse events; immune-related cutaneous adverse events; ustekinumab.
Conflict of interest statement
S.G., T.M., A.M., and S.D. have no disclosures to report. D.M.F. has received consulting fees from AzurRx, Equillium, Gilead, Janssen, Mallinckrodt Pharmaceuticals, and OnQuality Pharmaceuticals. NJS is a Speaker/Travel Consultant for Merk, Exelixis, Graticule, MedNet, and DAVA Oncology and receives research funding from Aravive and Exelixis. M.E.L. has a consultant role with Johnson and Johnson, Novocure, QED, Bicara, Janssen, Novartis, F. Hoffmann-LaRoche AG, EMD Serono, AstraZeneca, Innovaderm, Deciphera, DFB, Azitra, Kintara, RBC/La Roche Posay, Trifecta, Varsona, Genentech, Loxo, Seattle Genetics, Lutris, On Quality, Azitra, Roche, Oncoderm, NCODA, and Apricity. M.E.L. receives research funding from Lutris, Paxman, Novocure, J&J, US Biotest, OQL, Novartis, and AstraZeneca.
Figures
References
-
- Hellmann M.D., Paz-Ares L., Bernabe Caro R., Zurawski B., Kim S.W., Carcereny Costa E., Park K., Alexandru A., Lupinacci L., de la Mora Jimenez E., et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019;381:2020–2031. doi: 10.1056/NEJMoa1910231. - DOI - PubMed
-
- Shah N.J., Sura S.D., Shinde R., Shi J., Singhal P.K., Robert N.J., Vogelzang N.J., Perini R.F., Motzer R.J. Real-world Treatment Patterns and Clinical Outcomes for Metastatic Renal Cell Carcinoma in the Current Treatment Era. Eur. Urol. Open Sci. 2023;49:110–118. doi: 10.1016/j.euros.2022.12.015. - DOI - PMC - PubMed
-
- Wolchok J.D., Chiarion-Sileni V., Gonzalez R., Rutkowski P., Grob J.J., Cowey C.L., Lao C.D., Wagstaff J., Schadendorf D., Ferrucci P.F., et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

