Statistically Optimized Polymeric Buccal Films of Eletriptan Hydrobromide and Itopride Hydrochloride: An In Vivo Pharmacokinetic Study
- PMID: 38004417
- PMCID: PMC10674159
- DOI: 10.3390/ph16111551
Statistically Optimized Polymeric Buccal Films of Eletriptan Hydrobromide and Itopride Hydrochloride: An In Vivo Pharmacokinetic Study
Abstract
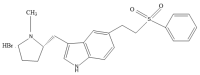
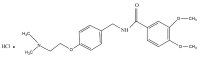
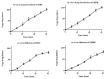
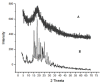
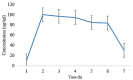
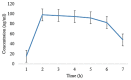
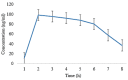
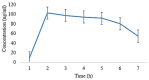
A migraine is a condition of severe headaches, causing a disturbance in the daily life of the patient. The current studies were designed to develop immediate-release polymeric buccal films of Eletriptan Hydrobromide (EHBR) and Itopride Hydrochloride (ITHC) to improve their bioavailability and, hence, improve compliance with the patients of migraines and its associated symptoms. The prepared films were evaluated for various in vitro parameters, including surface morphology, mechanical strength, disintegration test (DT), total dissolving time (TDT), drug release and drug permeation, etc., and in vivo pharmacokinetic parameters, such as area under curve (AUC), mean residence time (MRT), half-life (t1/2), time to reach maximum concentration (Tmax), and time to reach maximum concentration (Cmax). The outcomes have indicated the successful preparation of the films, as SEM has confirmed the smooth surface and uniform distribution of drugs throughout the polymer matrix. The films were found to be mechanically stable as indicated by folding endurance studies. Furthermore, the optimized formulations showed a DT of 13 ± 1 s and TDT of 42.6 ± 0.75 s, indicating prompt disintegration as well as the dissolution of the films. Albino rabbits were used for in vivo pharmacokinetics, and the outcomes were evident of improved pharmacokinetics. The drug was found to rapidly permeate across the buccal mucosa, leading to increased bioavailability of the drug: Cmax of 130 and 119 ng/mL of ITHC and EHBR, respectively, as compared to 96 (ITHC) and 90 ng/mL (EHBR) of oral solution. The conclusion can be drawn that possible reasons for the enhanced bioavailability could be the increased surface area in the form of buccal films, its rapid disintegration, and faster dissolution, which led toward the rapid absorption of the drug into the blood stream.
Keywords: buccal film; drug delivery; immediate dosage form; plasticizer; surfactant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Design and Evaluation of Instant Release Buccal Films of Empagliflozin: A Statistical Approach.ACS Omega. 2025 May 27;10(22):23492-23503. doi: 10.1021/acsomega.5c02082. eCollection 2025 Jun 10. ACS Omega. 2025. PMID: 40521519 Free PMC article.
-
Development of Tizanidine HCl-Meloxicam loaded mucoadhesive buccal films: In-vitro and in-vivo evaluation.PLoS One. 2018 Mar 22;13(3):e0194410. doi: 10.1371/journal.pone.0194410. eCollection 2018. PLoS One. 2018. PMID: 29566073 Free PMC article.
-
The Development of Eletriptan Hydrobromide Immediate Release Buccal Films Using Central Composite Rotatable Design: An In Vivo and In Vitro Approach.Polymers (Basel). 2022 Sep 23;14(19):3981. doi: 10.3390/polym14193981. Polymers (Basel). 2022. PMID: 36235932 Free PMC article.
-
Development and Evaluation of an Anti-Epileptic Oral Fast-Dissolving Film with Enhanced Dissolution and In vivo Permeation.Curr Drug Deliv. 2018;15(9):1294-1304. doi: 10.2174/1567201815666180723115600. Curr Drug Deliv. 2018. PMID: 30033870
-
Hexyl alginate derivative, an amphiphilic innovative buccal film-forming material of promising mechanical and release characteristics for the improvement of repaglinide bioavailability.Drug Des Devel Ther. 2019 Mar 21;13:925-940. doi: 10.2147/DDDT.S196425. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 30962675 Free PMC article.
Cited by
-
Toxicity and Toxicokinetics of a Four-Week Repeated Gavage of Levamisole in Male Beagle Dogs: A Good Laboratory Practice Study.Pharmaceuticals (Basel). 2024 Jan 22;17(1):141. doi: 10.3390/ph17010141. Pharmaceuticals (Basel). 2024. PMID: 38276014 Free PMC article.
-
Design and Evaluation of Instant Release Buccal Films of Empagliflozin: A Statistical Approach.ACS Omega. 2025 May 27;10(22):23492-23503. doi: 10.1021/acsomega.5c02082. eCollection 2025 Jun 10. ACS Omega. 2025. PMID: 40521519 Free PMC article.
References
-
- Goel A.N., Long J.L. Chapter 2—The Oral Cavity. In: Chhetri D.K., Dewan K., editors. Dysphagia Evaluation and Management in Otolaryngology. Elsevier; Amsterdam, The Netherlands: 2019. pp. 5–12.
-
- Arya A., Chandra A., Sharma V., Pathak K. Fast dissolving oral films: An innovative drug delivery system and dosage form. Int. J. ChemTech Res. 2010;2:576–583.
-
- Jagtap V.D. Buccal Film—A Review on Novel Drug Delivery System. Int. J. Res. Rev. 2020;7:17–28.
-
- Huanbutta K., Sangnim T. Bioadhesives in Drug Delivery. Scrivener Publishing; Beverly, MA, USA: 2020. Bioadhesive Films for Drug Delivery Systems; pp. 99–122.
Grants and funding
LinkOut - more resources
Full Text Sources

