Enhancing Tamoxifen Therapy with α-Mangostin: Synergistic Antiproliferative Effects on Breast Cancer Cells and Potential Reduced Endometrial Impact
- PMID: 38004441
- PMCID: PMC10675669
- DOI: 10.3390/ph16111576
Enhancing Tamoxifen Therapy with α-Mangostin: Synergistic Antiproliferative Effects on Breast Cancer Cells and Potential Reduced Endometrial Impact
Abstract
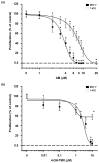
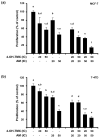
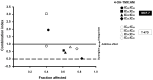
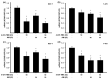
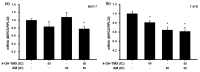
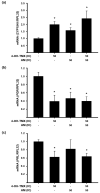
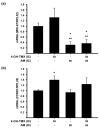
Breast cancer is the most prevalent neoplasia among women worldwide. For the estrogen receptor-positive (ER+) phenotype, tamoxifen is the standard hormonal therapy; however, it carries the risk of promoting endometrial carcinoma. Hence, we aimed to evaluate the antiproliferative effect of the phytochemical α-mangostin (AM) as a co-adjuvant alongside tamoxifen on breast cancer cells to improve its efficacy while reducing its adverse effects on endometrium. For this, ER+ breast cancer cells (MCF-7 and T-47D) and endometrial cells (N30) were treated with AM, 4-hydroxytamoxifen (4-OH-TMX), and their combination. Cell proliferation was evaluated using sulforhodamine B assay, and the pharmacological interaction was determined through the combination index and the dose reduction index calculation. The genes KCNH1, CCDN1, MKI67, and BIRC5 were amplified by real-time PCR as indicators of oncogenesis, cell cycle progression, cell proliferation, and apoptosis, respectively. Additionally, genes involved in ER signaling were analyzed. In breast cancer cells, the combination of AM with 4-OH-TMX showed a synergistic antiproliferative effect and favorable dose reduction. AM and 4-OH-TMX decreased KCNH1, CCND1, and BIRC5 gene expression. In endometrial cells, AM decreased MKI-67 gene expression, while it reverted the 4-OH-TMX-dependent CCND1 upregulation. This study establishes the benefits of incorporating AM as a co-adjuvant for first-line ER+ breast cancer therapy.
Keywords: KCNH1; breast cancer; combination index; endometrium cells; synergism; tamoxifen; α-mangostin.
Conflict of interest statement
The authors declare that no potential conflict of interest exist.
Figures
Similar articles
-
During hormone depletion or tamoxifen treatment of breast cancer cells the estrogen receptor apoprotein supports cell cycling through the retinoic acid receptor α1 apoprotein.Breast Cancer Res. 2011 Feb 7;13(1):R18. doi: 10.1186/bcr2827. Breast Cancer Res. 2011. PMID: 21299862 Free PMC article.
-
Selenium disrupts estrogen receptor (alpha) signaling and potentiates tamoxifen antagonism in endometrial cancer cells and tamoxifen-resistant breast cancer cells.Mol Cancer Ther. 2005 Aug;4(8):1239-49. doi: 10.1158/1535-7163.MCT-05-0046. Mol Cancer Ther. 2005. PMID: 16093440
-
EM-652 (SCH 57068), a third generation SERM acting as pure antiestrogen in the mammary gland and endometrium.J Steroid Biochem Mol Biol. 1999 Apr-Jun;69(1-6):51-84. doi: 10.1016/s0960-0760(99)00065-5. J Steroid Biochem Mol Biol. 1999. PMID: 10418981 Review.
-
Formulation of Anti-miR-21 and 4-Hydroxytamoxifen Co-loaded Biodegradable Polymer Nanoparticles and Their Antiproliferative Effect on Breast Cancer Cells.Mol Pharm. 2015 Jun 1;12(6):2080-92. doi: 10.1021/mp500852s. Epub 2015 Apr 28. Mol Pharm. 2015. PMID: 25880495 Free PMC article.
-
Targeting fatty acid synthase in breast and endometrial cancer: An alternative to selective estrogen receptor modulators?Endocrinology. 2006 Sep;147(9):4056-66. doi: 10.1210/en.2006-0486. Epub 2006 Jun 29. Endocrinology. 2006. PMID: 16809439 Review.
Cited by
-
Exploring the antineoplastic potential of α-mangostin in breast cancer.Nat Prod Bioprospect. 2025 Jul 3;15(1):43. doi: 10.1007/s13659-025-00528-5. Nat Prod Bioprospect. 2025. PMID: 40608162 Free PMC article. Review.
-
The Excellent Chemical Interaction Properties of Poloxamer and Pullulan with Alpha Mangostin on Amorphous Solid Dispersion System: Molecular Dynamics Simulation.Polymers (Basel). 2024 Oct 31;16(21):3065. doi: 10.3390/polym16213065. Polymers (Basel). 2024. PMID: 39518274 Free PMC article.
-
Improving Tamoxifen Performance in Inducing Apoptosis and Hepatoprotection by Loading on a Dual Nanomagnetic Targeting System.Anticancer Agents Med Chem. 2024;24(13):1016-1028. doi: 10.2174/0118715206289666240423091244. Anticancer Agents Med Chem. 2024. PMID: 38685808
References
-
- Sotiriou C., Wirapati P., Loi S., Harris A., Fox S., Smeds J., Nordgren H., Farmer P., Praz V., Haibe-Kains B., et al. Gene expression profiling in breast cancer: Understanding the molecular basis of histologic grade to improve prognosis. J. Natl. Cancer Inst. 2006;98:262–272. doi: 10.1093/jnci/djj052. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

