Comparative Analysis of the Physicochemical and Biological Characteristics of Freeze-Dried PEGylated Cationic Solid Lipid Nanoparticles
- PMID: 38004448
- PMCID: PMC10675625
- DOI: 10.3390/ph16111583
Comparative Analysis of the Physicochemical and Biological Characteristics of Freeze-Dried PEGylated Cationic Solid Lipid Nanoparticles
Abstract
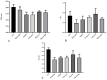
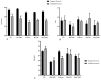
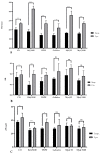
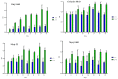
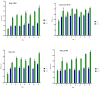
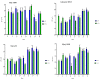
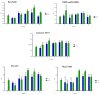
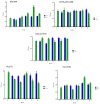
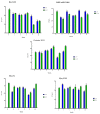
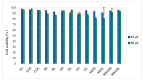
Cationic solid-lipid nanoparticles (cSLNs) have become a promising tool for gene and RNA therapies. PEGylation (PEG) is crucial in enhancing particle stability and protection. We evaluated the impact of PEG on the physicochemical and biological characteristics of cholesteryl-oleate cSLNs (CO-cSLNs). Several parameters were analyzed, including the particle size, polydispersity index, zeta potential, shape, stability, cytotoxicity, and loading efficiency. Five different formulations with specific PEGs were developed and compared in both suspended and freeze-dried states. Small, homogeneous, and cationic suspended nanoparticles were obtained, with the Gelucire 50/13 (PEG-32 hydrogenated palm glycerides; Gelucire) and DSPE-mPEG2000 (1,2-distearoyl-phosphatidylethanolamine-methyl-polyethyleneglycol conjungate-2000; DSPE) formulations exhibiting the smallest particle size (~170 nm). Monodisperse populations of freeze-dried nanoparticles were also achieved, with particle sizes ranging from 200 to 300 nm and Z potential values of 30-35 mV. Notably, Gelucire again produced the smallest particle size (211.1 ± 22.4), while the DSPE and Myrj S100 (polyoxyethylene (100) stearate; PEG-100 Stearate) formulations had similar particle sizes to CO-cSLNs (~235 nm). The obtained PEGylated nanoparticles showed suitable properties: they were nontoxic, had acceptable morphology, were capable of forming SLNplexes, and were stable in both suspended and lyophilized states. These PEG-cSLNs are a potential resource for in vivo assays and have the advantage of employing cost-effective PEGs. Optimizing the lyophilization process and standardizing parameters are also recommended to maintain nanoparticle integrity.
Keywords: PEG; SLNplexes; cSLNs; cationic solid lipid nanoparticles; freeze-drying; gene therapy; lyophilization; morphology; poly(ethylene glycol); stability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Development of Glycyrrhetinic Acid and Folate Modified Cantharidin Loaded Solid Lipid Nanoparticles for Targeting Hepatocellular Carcinoma.Molecules. 2022 Oct 11;27(20):6786. doi: 10.3390/molecules27206786. Molecules. 2022. PMID: 36296377 Free PMC article.
-
A promising approach to develop nanostructured lipid carriers from solid lipid nanoparticles: preparation, characterization, cytotoxicity and nucleic acid binding ability.Pharm Dev Technol. 2020 Oct;25(8):936-948. doi: 10.1080/10837450.2020.1759630. Epub 2020 May 14. Pharm Dev Technol. 2020. PMID: 32315242
-
Lyophilization and stability of antibody-conjugated mesoporous silica nanoparticle with cationic polymer and PEG for siRNA delivery.Int J Nanomedicine. 2018 Jul 10;13:4015-4027. doi: 10.2147/IJN.S164393. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30022824 Free PMC article.
-
Preparation and Characterization of Cationic PLA-PEG Nanoparticles for Delivery of Plasmid DNA.Nanoscale Res Lett. 2009 May 21;4(9):982-992. doi: 10.1007/s11671-009-9345-3. Nanoscale Res Lett. 2009. PMID: 20596550 Free PMC article.
-
Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review.
Cited by
-
PEGylation-Dependent Cell Uptake of Lipid Nanoparticles Revealed by Spatiotemporal Correlation Spectroscopy.ACS Pharmacol Transl Sci. 2024 Sep 5;7(10):3004-3010. doi: 10.1021/acsptsci.4c00419. eCollection 2024 Oct 11. ACS Pharmacol Transl Sci. 2024. PMID: 39421655
References
-
- dos Santos Coura R., Nardi N.B. A role for adeno-associated viral vectors in gene therapy. Genet. Mol. Biol. 2008;31:1–11. doi: 10.1590/S1415-47572008000100001. - DOI

