PBPK Modeling of Azithromycin Systemic Exposure in a Roux-en-Y Gastric Bypass Surgery Patient Population
- PMID: 38004500
- PMCID: PMC10674169
- DOI: 10.3390/pharmaceutics15112520
PBPK Modeling of Azithromycin Systemic Exposure in a Roux-en-Y Gastric Bypass Surgery Patient Population
Abstract
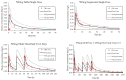
In this investigation, PBPK modeling using the Simcyp® Simulator was performed to evaluate whether Roux-en-Y gastric bypass (RYGB) surgery impacts the oral absorption and bioavailability of azithromycin. An RYGB surgery patient population was adapted from the published literature and verified using the same probe medications, atorvastatin and midazolam. Next, a PBPK model of azithromycin was constructed to simulate changes in systemic drug exposure after the administration of different oral formulations (tablet, suspension) to patients pre- and post-RYGB surgery using the developed and verified population model. Clinically observed changes in azithromycin systemic exposure post-surgery following oral administration (single-dose tablet formulation) were captured using PBPK modeling based on the comparison of model-predicted exposure metrics (Cmax, AUC) to published clinical data. Model simulations predicted a 30% reduction in steady-state AUC after surgery for three- and five-day multiple dose regimens of an azithromycin tablet formulation. The relative bioavailability of a suspension formulation was 1.5-fold higher than the tablet formulation after multiple dosing. The changes in systemic exposure observed after surgery were used to evaluate the clinical efficacy of azithromycin against two of the most common pathogens causing community acquired pneumonia based on the corresponding AUC24/MIC pharmacodynamic endpoint. The results suggest lower bioavailability of the tablet formulation post-surgery may impact clinical efficacy. Overall, the research demonstrates the potential of a PBPK modeling approach as a framework to optimize oral drug therapy in patients post-RYGB surgery.
Keywords: PBPK modeling; Roux-en-Y; azithromycin; pharmacokinetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Physiologically-Based Pharmacokinetic Model on the Oral Drug Absorption in Roux-en-Y Gastric Bypass Bariatric Patients: Amoxicillin Tablet and Suspension.Mol Pharm. 2019 Dec 2;16(12):5025-5034. doi: 10.1021/acs.molpharmaceut.9b00870. Epub 2019 Nov 18. Mol Pharm. 2019. PMID: 31721592
-
PBPK modeling of CYP3A and P-gp substrates to predict drug-drug interactions in patients undergoing Roux-en-Y gastric bypass surgery.J Pharmacokinet Pharmacodyn. 2020 Oct;47(5):493-512. doi: 10.1007/s10928-020-09701-4. Epub 2020 Jul 24. J Pharmacokinet Pharmacodyn. 2020. PMID: 32710209
-
Drug disposition and modelling before and after gastric bypass: immediate and controlled-release metoprolol formulations.Br J Clin Pharmacol. 2015 Nov;80(5):1021-30. doi: 10.1111/bcp.12666. Epub 2015 Jul 6. Br J Clin Pharmacol. 2015. PMID: 25917170 Free PMC article. Clinical Trial.
-
Impaired oral absorption of methylphenidate after Roux-en-Y gastric bypass.Surg Obes Relat Dis. 2017 Jul;13(7):1245-1247. doi: 10.1016/j.soard.2017.03.003. Epub 2017 Mar 9. Surg Obes Relat Dis. 2017. PMID: 28420586 Review.
-
Impact of Roux-en-Y Gastric Bypass Surgery on Pharmacokinetics of Administered Drugs: Implications and Perspectives.Am J Ther. 2016 Nov/Dec;23(6):e1826-e1838. doi: 10.1097/MJT.0000000000000317. Am J Ther. 2016. PMID: 26398718 Review.
Cited by
-
Physiologically Based Pharmacokinetic Modeling for Predicting Drug Levels After Bariatric Surgery: Vardenafil Exposure Before vs. After Gastric Sleeve/Bypass.Biomolecules. 2025 Jul 7;15(7):975. doi: 10.3390/biom15070975. Biomolecules. 2025. PMID: 40723847 Free PMC article.
References
-
- Darwich A.S., Henderson K., Burgin A., Ward N., Whittam J., Ammori B.J., Ashcroft D.M., Rostami-Hodjegan A. Trends in oral drug bioavailability following bariatric surgery: Examining the variable extent of impact on exposure of different drug classes. Br. J. Clin. Pharmacol. 2012;74:774–787. doi: 10.1111/j.1365-2125.2012.04284.x. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

