Population Pharmacodynamic Modelling of the CD19+ Suppression Effects of Rituximab in Paediatric Patients with Neurological and Autoimmune Diseases
- PMID: 38004515
- PMCID: PMC10674351
- DOI: 10.3390/pharmaceutics15112534
Population Pharmacodynamic Modelling of the CD19+ Suppression Effects of Rituximab in Paediatric Patients with Neurological and Autoimmune Diseases
Abstract
Background: Limited pharmacotherapy and the failure of conventional treatments in complex pathologies in children lead to increased off-label use of rituximab. We aimed to characterize the time course of CD19+ B lymphocytes (CD19+) under treatment with intravenous rituximab in children with neurologic and autoimmune diseases and to evaluate the impact of covariates (i.e., demographics, diagnosis and substitution between innovator and biosimilar product) on rituximab pharmacodynamics and disease activity.
Methods: Pre- and post-drug infusion CD19+ in peripheral blood were prospectively registered. A population pharmacodynamic model describing the time course of CD19+ was developed with NONMEM v7.4. Simulations of three different rituximab regimens were performed to assess the impact on CD19+. Logistic regression analysis was performed to identify predictors of clinical response recorded through disease activity scores.
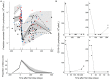
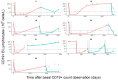
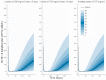
Results: 281 measurements of CD19+ lymphocyte counts obtained from 63 children with neurologic (n = 36) and autoimmune (n = 27) diseases were available. The time course of CD19+ was described with a turn-over model in which the balance between synthesis and degradation rates is disrupted by rituximab, increasing the latter process. The model predicts half-lives (percent coefficient of variation, CV(%)) of rituximab and CD19+ of 11.6 days (17%) and 173.3 days (22%), respectively. No statistically significant effect was found between any of the studied covariates and model parameters (p > 0.05). Simulations of different regimens showed no clinically significant differences in terms of CD19+ repopulation times. A trend towards a lack of clinical response was observed in patients with lower CD19+ repopulation times and higher areas under the CD19+ versus time curve.
Conclusions: Rituximab pharmacodynamics was described in a real-world setting in children suffering from autoimmune and neurologic diseases. Diagnosis, substitution between innovator rituximab and its biosimilars or type of regimen did not affect rituximab-induced depletion of CD19+ nor the clinical response in this cohort of patients. According to this study, rituximab frequency and dosage may be chosen based on clinical convenience or safety reasons without affecting CD19+ repopulation times. Further studies in larger populations are required to confirm these results.
Keywords: NONMEM; biosimilar pharmaceuticals; paediatrics; pharmacodynamics; rituximab.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Pharmacodynamics of rituximab on B lymphocytes in paediatric patients with autoimmune diseases.Br J Clin Pharmacol. 2019 Aug;85(8):1790-1797. doi: 10.1111/bcp.13970. Epub 2019 Jun 20. Br J Clin Pharmacol. 2019. PMID: 31026092 Free PMC article.
-
Using real-world data to inform dosing strategies of rituximab for pediatric patients with frequent-relapsing or steroid-dependent nephrotic syndrome: a prospective pharmacokinetic-pharmacodynamic study.Front Pharmacol. 2024 Jan 9;14:1319744. doi: 10.3389/fphar.2023.1319744. eCollection 2023. Front Pharmacol. 2024. PMID: 38264525 Free PMC article.
-
Pharmacokinetic-pharmacodynamic modelling in a clinical pilot study of rituximab in multiple sclerosis: Towards personalized dosing interval.Br J Clin Pharmacol. 2025 Jun 14. doi: 10.1002/bcp.70136. Online ahead of print. Br J Clin Pharmacol. 2025. PMID: 40515509
-
Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia.Drugs. 2003;63(8):803-43. doi: 10.2165/00003495-200363080-00005. Drugs. 2003. PMID: 12662126 Review.
-
Current evidence of rituximab in the treatment of multiple sclerosis.Mult Scler Relat Disord. 2023 Jul;75:104729. doi: 10.1016/j.msard.2023.104729. Epub 2023 Apr 23. Mult Scler Relat Disord. 2023. PMID: 37148577 Review.
Cited by
-
Kinetic-pharmacodynamic model to predict post-rituximab B-cell repletion as a predictor of relapse in pediatric idiopathic nephrotic syndrome.Front Pharmacol. 2025 Jan 7;15:1526936. doi: 10.3389/fphar.2024.1526936. eCollection 2024. Front Pharmacol. 2025. PMID: 39840094 Free PMC article.
-
The Role of Pharmacometrics in Advancing the Therapies for Autoimmune Diseases.Pharmaceutics. 2024 Dec 5;16(12):1559. doi: 10.3390/pharmaceutics16121559. Pharmaceutics. 2024. PMID: 39771538 Free PMC article. Review.
References
-
- Kasi P.M., Tawbi H.A., Oddis C.V., Kulkarni H.S. Clinical review: Serious adverse events associated with the use of rituximab—A critical care perspective. [(accessed on 16 June 2023)];Crit. Care. 2012 16:231. doi: 10.1186/cc11304. Available online: https://pubmed.ncbi.nlm.nih.gov/22967460/ - DOI - PMC - PubMed
-
- Micromedex-Solutions Rituximab. 2023. [(accessed on 16 August 2023)]. Available online: http://www.micromedexsolutions.com.
-
- Tenembaum S., Yeh E.A. Pediatric NMOSD: A Review and Position Statement on Approach to Work-Up and Diagnosis. [(accessed on 16 June 2023)];Front. Pediatr. 2020 8:339. doi: 10.3389/fped.2020.00339. Available online: https://pubmed.ncbi.nlm.nih.gov/32671002/ - DOI - PMC - PubMed
-
- Genberg H., Hansson A., Wernerson A., Wennberg L., Tydén G. Pharmacodynamics of rituximab in kidney allotransplantation. [(accessed on 16 June 2023)];Am. J. Transplant. 2006 6:2418–2428. doi: 10.1111/j.1600-6143.2006.01497.x. Available online: https://pubmed.ncbi.nlm.nih.gov/16925569/ - DOI - PubMed
-
- Ellrichmann G., Bolz J., Peschke M., Duscha A., Hellwig K., Lee D.H., Linker R.A., Gold R., Haghikia A. Peripheral CD19+ B-cell counts and infusion intervals as a surrogate for long-term B-cell depleting therapy in multiple sclerosis and neuromyelitis optica/neuromyelitis optica spectrum disorders. [(accessed on 16 June 2023)];J. Neurol. 2019 266:57–67. doi: 10.1007/s00415-018-9092-4. Available online: https://pubmed.ncbi.nlm.nih.gov/30377816/ - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

