Photodynamic Therapy for X-ray-Induced Radiation-Resistant Cancer Cells
- PMID: 38004516
- PMCID: PMC10674178
- DOI: 10.3390/pharmaceutics15112536
Photodynamic Therapy for X-ray-Induced Radiation-Resistant Cancer Cells
Abstract
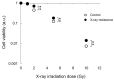
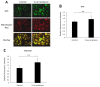
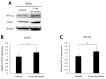
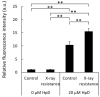
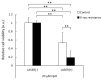
Radiotherapy, in which X-rays are commonly used, is one of the most effective procedures for treating cancer. However, some cancer cells become resistant to radiation therapy, leading to poor prognosis. Therefore, a new therapeutic method is required to prevent cancer cells from acquiring radiation resistance. Photodynamic therapy (PDT) is a cancer treatment that uses photosensitizers, such as porphyrin compounds, and low-powered laser irradiation. We previously reported that reactive oxygen species (ROS) derived from mitochondria induce the expression of a porphyrin transporter (HCP1) and that laser irradiation enhances the cytotoxic effect. In addition, X-ray irradiation induces the production of mitochondrial ROS. Therefore, radioresistant cancer cells established with continuous X-ray irradiation would also overexpress ROS, and photodynamic therapy could be an effective therapeutic method. In this study, we established radioresistant cancer cells and examined the therapeutic effects and mechanisms with photodynamic therapy. We confirmed that X-ray-resistant cells showed overgeneration of mitochondrial ROS and elevated expression of HCP1, which led to the active accumulation of porphyrin and an increase in cytotoxicity with laser irradiation. Thus, photodynamic therapy is a promising treatment for X-ray-resistant cancers.
Keywords: HCP1; radioresistance; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Enhancement of cytotoxic effects with ALA-PDT on treatment of radioresistant cancer cells.J Clin Biochem Nutr. 2024 Jan;74(1):17-21. doi: 10.3164/jcbn.23-79. Epub 2023 Oct 3. J Clin Biochem Nutr. 2024. PMID: 38292126 Free PMC article.
-
Acetic acid enhances the effect of photodynamic therapy in gastric cancer cells via the production of reactive oxygen species.J Clin Biochem Nutr. 2022 Nov;71(3):206-211. doi: 10.3164/jcbn.22-34. Epub 2022 Aug 4. J Clin Biochem Nutr. 2022. PMID: 36447491 Free PMC article.
-
The Cisplatin-Derived Increase of Mitochondrial Reactive Oxygen Species Enhances the Effectiveness of Photodynamic Therapy via Transporter Regulation.Cells. 2019 Aug 17;8(8):918. doi: 10.3390/cells8080918. Cells. 2019. PMID: 31426474 Free PMC article.
-
Recent Progress and Trends in X-ray-Induced Photodynamic Therapy with Low Radiation Doses.ACS Nano. 2022 Dec 27;16(12):19691-19721. doi: 10.1021/acsnano.2c07286. Epub 2022 Nov 15. ACS Nano. 2022. PMID: 36378555 Review.
-
Difference in Acquired Radioresistance Induction Between Repeated Photon and Particle Irradiation.Front Oncol. 2019 Nov 12;9:1213. doi: 10.3389/fonc.2019.01213. eCollection 2019. Front Oncol. 2019. PMID: 31799186 Free PMC article. Review.
Cited by
-
Radiation-Activated Cobalt-Based Zeolite Imidazolate Frameworks for Tumor Multitherapy.Biomater Res. 2025 Apr 15;29:0164. doi: 10.34133/bmr.0164. eCollection 2025. Biomater Res. 2025. PMID: 40236956 Free PMC article.
-
Advancements and emerging trends in photodynamic therapy: innovations in cancer treatment and beyond.Photochem Photobiol Sci. 2025 Jul 24. doi: 10.1007/s43630-025-00765-0. Online ahead of print. Photochem Photobiol Sci. 2025. PMID: 40705276 Review.
-
Activatable Photosensitizers: From Fundamental Principles to Advanced Designs.Angew Chem Int Ed Engl. 2025 Apr 7;64(15):e202423348. doi: 10.1002/anie.202423348. Epub 2025 Feb 21. Angew Chem Int Ed Engl. 2025. PMID: 39899458 Free PMC article. Review.
References
-
- Toyoda K., Yasaka M., Iwade K., Nagata K., Koretsune Y., Sakamoto T., Uchiyama S., Gotoh J., Nagao T., Yamamoto M., et al. Dual antithrombotic therapy increases severe bleeding events in patients with stroke and cardiovascular disease: A prospective, multicenter, observational study. Stroke. 2008;39:1740–1745. doi: 10.1161/STROKEAHA.107.504993. - DOI - PubMed
-
- Louison S., Gabrielle P.H., Soudry A., Meillon C., Blanc J., Béal G., Arsène S., Le Mer Y., Berrod J.P., Kodjikian L., et al. Perioperative risk of bleeding with antithrombotic agents in macular surgery: A national, prospective, multicentre study. Acta Ophthalmol. 2020;98:e991–e997. doi: 10.1111/aos.14434. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

