Selegiline Modulates Lipid Metabolism by Activating AMPK Pathways of Epididymal White Adipose Tissues in HFD-Fed Obese Mice
- PMID: 38004519
- PMCID: PMC10675427
- DOI: 10.3390/pharmaceutics15112539
Selegiline Modulates Lipid Metabolism by Activating AMPK Pathways of Epididymal White Adipose Tissues in HFD-Fed Obese Mice
Abstract
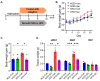
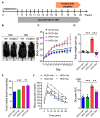
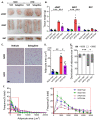
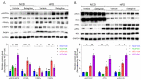
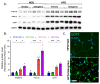
Obesity, as a major cause of many chronic diseases such as diabetes, cardiovascular disease, and cancer, is among the most serious health problems. Increased monoamine oxidase (MAO) activity has been observed in the adipose tissue of obese humans and animals. Although previous studies have already demonstrated the potential of MAO-B inhibitors as a treatment for this condition, the mechanism of their effect has been insufficiently elucidated. In this study, we investigated the anti-obesity effect of selegiline, a selective MAO-B inhibitor, using in vivo animal models. The effect was evaluated through an assessment of body energy homeostasis, glucose tolerance tests, and biochemical analysis. Pharmacological inhibition of MAO-B by selegiline was observed to reduce body weight and fat accumulation, and improved glucose metabolism without a corresponding change in food intake, in HFD-fed obese mice. We also observed that both the expression of adipogenenic markers, including C/EBPα and FABP4, and lipogenic markers such as pACC were significantly reduced in epididymal white adipose tissues (eWATs). Conversely, increased expression of lipolytic markers such as ATGL and pHSL and AMPK phosphorylation were noted. Treating obese mice with selegiline significantly increased expression levels of UCP1 and promoted eWAT browning, indicating increased energy expenditure. These results suggest that selegiline, by inhibiting MAO-B activity, is a potential anti-obesity treatment.
Keywords: adipogenesis; anti-obesity; eWAT browning; lipogenesis; lipolysis; selegiline.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Emodin Improves Glucose and Lipid Metabolism Disorders in Obese Mice via Activating Brown Adipose Tissue and Inducing Browning of White Adipose Tissue.Front Endocrinol (Lausanne). 2021 May 10;12:618037. doi: 10.3389/fendo.2021.618037. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34040579 Free PMC article.
-
Selegiline reduces adiposity induced by high-fat, high-sucrose diet in male rats.Br J Pharmacol. 2018 Sep;175(18):3713-3726. doi: 10.1111/bph.14437. Epub 2018 Aug 7. Br J Pharmacol. 2018. PMID: 29971762 Free PMC article.
-
Spirulina maxima Extract Reduces Obesity through Suppression of Adipogenesis and Activation of Browning in 3T3-L1 Cells and High-Fat Diet-Induced Obese Mice.Nutrients. 2018 Jun 1;10(6):712. doi: 10.3390/nu10060712. Nutrients. 2018. PMID: 29865208 Free PMC article.
-
AMP-Activated Protein Kinase (AMPK) Regulates Energy Metabolism through Modulating Thermogenesis in Adipose Tissue.Front Physiol. 2018 Feb 21;9:122. doi: 10.3389/fphys.2018.00122. eCollection 2018. Front Physiol. 2018. PMID: 29515462 Free PMC article.
-
Selegiline: a molecule with innovative potential.J Neural Transm (Vienna). 2020 May;127(5):831-842. doi: 10.1007/s00702-019-02082-0. Epub 2019 Sep 27. J Neural Transm (Vienna). 2020. PMID: 31562557 Free PMC article. Review.
Cited by
-
Abundance of a metabolically active subpopulation in dedifferentiated adipocytes inversely correlates with body mass index.Mol Metab. 2025 Jul;97:102161. doi: 10.1016/j.molmet.2025.102161. Epub 2025 May 8. Mol Metab. 2025. PMID: 40348015 Free PMC article.
-
Machine learning combining external validation to explore the immunopathogenesis of diabetic foot ulcer and predict therapeutic drugs.PLoS One. 2025 Aug 1;20(8):e0328906. doi: 10.1371/journal.pone.0328906. eCollection 2025. PLoS One. 2025. PMID: 40749079 Free PMC article.
References
-
- Zhang F., Ai W., Hu X., Meng Y., Yuan C., Su H., Wang L., Zhu X., Gao P., Shu G., et al. Phytol stimulates the browning of white adipocytes through the activation of AMP-activated protein kinase (AMPK) alpha in mice fed high-fat diet. Food Funct. 2018;9:2043–2050. doi: 10.1039/C7FO01817G. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

