Effect of Basic Amino Acids on Folic Acid Solubility
- PMID: 38004524
- PMCID: PMC10675447
- DOI: 10.3390/pharmaceutics15112544
Effect of Basic Amino Acids on Folic Acid Solubility
Abstract
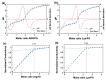
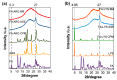
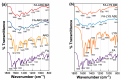
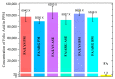
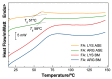
To prevent neural tube defects and other cardiovascular diseases in newborns, folic acid (FA) is recommended in pregnant women. A daily dose of 600 µg FA consumption is widely prescribed for women during pregnancy and 400 µg for women with childbearing potential. FA is a class IV compound according to the Biopharmaceutics Classification System (BCS) due to its low permeability (1.7 × 10-6 cm/s) and low solubility (1.6 mg/L); therefore, it must be administered via a formulation that enhances its solubility. Studies reported in the literature have proved that co-amorphization and salt formation of a poorly soluble drug with amino acids (AA) can significantly increase its solubility. Although arginine has been used with FA as a supplement, there is no information on the effect of basic AA (arginine and lysine) on the physical and chemical properties of FA-AA binary formulations. The present study implemented a conductimetric titration methodology to find the effective molar ratio to maximize FA solubility. The results showed that a 1:2.5 FA:AA molar ratio maximized solubility for arginine and lysine. Binary formulations were prepared using different methods, which led to an amorphous system confirmed by the presence of a glass transition, broad FTIR bands, and the absence of an X-ray diffraction pattern. Results of FA:AA (1:2.5) solubility increased in the range of 5500-6000 times compared with pure FA. In addition to solubility enhancement, the binary systems presented morphological properties that depend on the preparation method and whose consideration could be strategic for scaling purposes.
Keywords: amino acids; amorphous drug; arginine; ball milling; conductimetry; folic acid; lysine; solubility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Preparation and characterization of amorphous ciprofloxacin-amino acid salts.Eur J Pharm Biopharm. 2017 Dec;121:73-89. doi: 10.1016/j.ejpb.2017.09.009. Epub 2017 Sep 15. Eur J Pharm Biopharm. 2017. PMID: 28919470
-
Sinapic Acid Co-Amorphous Systems with Amino Acids for Improved Solubility and Antioxidant Activity.Int J Mol Sci. 2023 Mar 14;24(6):5533. doi: 10.3390/ijms24065533. Int J Mol Sci. 2023. PMID: 36982605 Free PMC article.
-
Preparation and characterization of spray-dried co-amorphous drug-amino acid salts.J Pharm Pharmacol. 2016 May;68(5):615-24. doi: 10.1111/jphp.12458. Epub 2015 Aug 5. J Pharm Pharmacol. 2016. PMID: 26245703
-
Amino acids as co-amorphous excipients for tackling the poor aqueous solubility of valsartan.Pharm Dev Technol. 2017 Feb;22(1):69-76. doi: 10.3109/10837450.2016.1163390. Epub 2016 Apr 6. Pharm Dev Technol. 2017. PMID: 27050301
-
Biopharmaceutical classification of poorly soluble drugs with respect to "enabling formulations".Eur J Pharm Sci. 2013 Sep 27;50(1):8-16. doi: 10.1016/j.ejps.2013.04.002. Epub 2013 Apr 11. Eur J Pharm Sci. 2013. PMID: 23583787 Review.
Cited by
-
Cutting-Edge Approaches in the Co-Amorphization Process.Pharmaceutics. 2025 Jun 29;17(7):850. doi: 10.3390/pharmaceutics17070850. Pharmaceutics. 2025. PMID: 40733059 Free PMC article. Review.
References
-
- Batinić P.M., Đorđević V.B., Stevanović S.I., Balanč B.D., Marković S.B., Luković N.D., Mijin D.Ž., Bugarski B.M. Formulation and Characterization of Novel Liposomes Containing Histidine for Encapsulation of a Poorly Soluble Vitamin. J. Drug Deliv. Sci. Technol. 2020;59:101920. doi: 10.1016/j.jddst.2020.101920. - DOI
-
- Higdon, J. (Instituto L.P. Folato | Linus Pauling Institute | Oregon State University) [(accessed on 19 April 2022)]. Available online: https://lpi.oregonstate.edu/es/mic/vitaminas/folato#determinacion-IDR.
-
- Braga D., Chelazzi L., Grepioni F., Maschio L., Nanna S., Taddei P. Folic Acid in the Solid State: A Synergistic Computational, Spectroscopic, and Structural Approach. Cryst. Growth Des. 2016;16:2218–2224. doi: 10.1021/acs.cgd.6b00043. - DOI
LinkOut - more resources
Full Text Sources

