The Role of Five-Membered Heterocycles in the Molecular Structure of Antibacterial Drugs Used in Therapy
- PMID: 38004534
- PMCID: PMC10675556
- DOI: 10.3390/pharmaceutics15112554
The Role of Five-Membered Heterocycles in the Molecular Structure of Antibacterial Drugs Used in Therapy
Abstract
Five-membered heterocycles are essential structural components in various antibacterial drugs; the physicochemical properties of a five-membered heterocycle can play a crucial role in determining the biological activity of an antibacterial drug. These properties can affect the drug's activity spectrum, potency, and pharmacokinetic and toxicological properties. Using scientific databases, we identified and discussed the antibacterials used in therapy, containing five-membered heterocycles in their molecular structure. The identified five-membered heterocycles used in antibacterial design contain one to four heteroatoms (nitrogen, oxygen, and sulfur). Antibacterials containing five-membered heterocycles were discussed, highlighting the biological properties imprinted by the targeted heterocycle. In some antibacterials, heterocycles with five atoms are pharmacophores responsible for their specific antibacterial activity. As pharmacophores, these heterocycles help design new medicinal molecules, improving their potency and selectivity and comprehending the structure-activity relationship of antibiotics. Unfortunately, particular heterocycles can also affect the drug's potential toxicity. The review extensively presents the most successful five-atom heterocycles used to design antibacterial essential medicines. Understanding and optimizing the intrinsic characteristics of a five-membered heterocycle can help the development of antibacterial drugs with improved activity, pharmacokinetic profile, and safety.
Keywords: antibacterials; antibiotics; biological activity; drug design; drug discovery; five-membered heterocycles; heterocycles; nitrogen heterocycles; oxygen heterocycles; sulfur heterocycles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

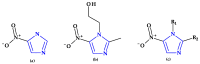
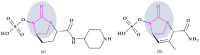
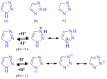
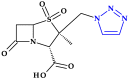

Similar articles
-
Unlocking the Potential of Pyrrole: Recent Advances in New Pyrrole-Containing Compounds with Antibacterial Potential.Int J Mol Sci. 2024 Nov 29;25(23):12873. doi: 10.3390/ijms252312873. Int J Mol Sci. 2024. PMID: 39684580 Free PMC article. Review.
-
Significance of Five-Membered Heterocycles in Human Histone Deacetylase Inhibitors.Molecules. 2023 Jul 27;28(15):5686. doi: 10.3390/molecules28155686. Molecules. 2023. PMID: 37570656 Free PMC article. Review.
-
Quantum chemical study of the structure and thermochemistry of the five-membered nitrogen-containing heterocycles and their anions and radicals.J Phys Chem A. 2006 Dec 28;110(51):13979-88. doi: 10.1021/jp065150w. J Phys Chem A. 2006. PMID: 17181359
-
Oxazole and isoxazole-containing pharmaceuticals: targets, pharmacological activities, and their SAR studies.RSC Med Chem. 2025 Feb 19. doi: 10.1039/d4md00777h. Online ahead of print. RSC Med Chem. 2025. PMID: 40008190 Free PMC article. Review.
-
Recent advances on anticancer and antimicrobial activities of directly-fluorinated five-membered heterocycles and their benzo-fused systems.RSC Adv. 2024 Jun 19;14(28):19752-19779. doi: 10.1039/d4ra01387e. eCollection 2024 Jun 18. RSC Adv. 2024. PMID: 38899036 Free PMC article. Review.
Cited by
-
Unlocking the Potential of Pyrrole: Recent Advances in New Pyrrole-Containing Compounds with Antibacterial Potential.Int J Mol Sci. 2024 Nov 29;25(23):12873. doi: 10.3390/ijms252312873. Int J Mol Sci. 2024. PMID: 39684580 Free PMC article. Review.
-
3,3'-((3-Hydroxyphenyl)azanediyl)dipropionic Acid Derivatives as a Promising Scaffold Against Drug-Resistant Pathogens and Chemotherapy-Resistant Cancer.Pathogens. 2025 May 15;14(5):484. doi: 10.3390/pathogens14050484. Pathogens. 2025. PMID: 40430804 Free PMC article.
-
Insights into bacterial interactions: Comparing fluorine-containing 1,2,4-triazoles to antibiotics using molecular docking and molecular dynamics approaches.Heliyon. 2024 Sep 6;10(17):e37538. doi: 10.1016/j.heliyon.2024.e37538. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39290291 Free PMC article.
-
Recent Developments of 1,3,4-Thiadiazole Compounds as Anticancer Agents.Pharmaceuticals (Basel). 2025 Apr 16;18(4):580. doi: 10.3390/ph18040580. Pharmaceuticals (Basel). 2025. PMID: 40284015 Free PMC article. Review.
-
Non-fused Pyrimidine Derivatives as Potential Pharmacological Entities: A Review.Curr Top Med Chem. 2025;25(9):1032-1068. doi: 10.2174/0115680266317088240924205745. Curr Top Med Chem. 2025. PMID: 39410888 Review.
References
-
- Gupta R.R., Kumar M., Gupta V. Heterocyclic Chemistry: Volume II: Five-Membered Heterocycles. Springer Science & Business Media; Berlin/Heidelberg, Germany: 2013.
-
- Gomtsyan A. Heterocycles in Drugs and Drug Discovery. Chem. Heterocycl. Comp. 2012;48:7–10. doi: 10.1007/s10593-012-0960-z. - DOI
-
- Barreca M., Spanò V., Rocca R., Bivacqua R., Gualtieri G., Raimondi M.V., Gaudio E., Bortolozzi R., Manfreda L., Bai R., et al. Identification of Pyrrolo [3′,4′:3,4]Cyclohepta[1,2-d][1,2]Oxazoles as Promising New Candidates for the Treatment of Lymphomas. Eur. J. Med. Chem. 2023;254:115372. doi: 10.1016/j.ejmech.2023.115372. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

