Development of a Novel Peptide with Antimicrobial and Mineralising Properties for Caries Management
- PMID: 38004539
- PMCID: PMC10675526
- DOI: 10.3390/pharmaceutics15112560
Development of a Novel Peptide with Antimicrobial and Mineralising Properties for Caries Management
Abstract
The purpose of the study is to develop a novel peptide for caries management. Gallic-Acid-Polyphemusin-I (GAPI) was synthesised by grafting Polyphemusin I (PI) and gallic acid (GA). Biocompatibility was evaluated using a Cell Counting Kit-8 Assay. Antimicrobial properties were assessed using minimum inhibitory concentration (MIC) and minimum bactericidal/fungicidal concentration (MBC/MFC). The bacterial and fungal morphology after GAPI treatment was investigated using transmission electron microscopy (TEM). The architecture of a consortium biofilm consisting of Streptococcus mutans, Lacticaseibacillus casei and Candida albicans was evaluated using scanning electron microscopy (SEM) and confocal laser scanning microscopy. The growth kinetics of the biofilm was examined using a propidium monoazide-quantitative polymerase chain reaction. The surface and calcium-to-phosphorus molar ratio of GAPI-treated enamel after pH cycling were examined with SEM and energy-dispersive X-ray spectroscopy. Enamel crystal characteristics were analysed using X-ray diffraction. Lesion depths representing the enamel's mineral loss were assessed using micro-computed tomography. The MIC of GAPI against S. mutans, L. casei and C. albicans were 40 μM, 40 μM and 20 μM, respectively. GAPI destroyed the biofilm's three-dimensional structure and inhibited the growth of the biofilm. SEM showed that enamel treated with GAPI had a relatively smooth surface compared to that treated with water. The calcium-to-phosphorus molar ratio of enamel treated with GAPI was higher than that of the control. The lesion depths and mineral loss of the GAPI-treated enamel were less than the control. The crystallinity of the GAPI-treated enamel was higher than the control. This study developed a biocompatible, mineralising and antimicrobial peptide GAPI, which may have potential as an anti-caries agent.
Keywords: antimicrobial; caries; mineralisation; peptides; prevention.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
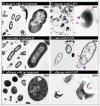
 Abnormal cell morphology; ↖ cytoplasmic clear zone;
Abnormal cell morphology; ↖ cytoplasmic clear zone;  disrupted membrane/cell wall; and
disrupted membrane/cell wall; and  cytoplasmic content leakage.
cytoplasmic content leakage.

Similar articles
-
Remineralising enamel caries with a novel peptide: An in vitro study.J Dent. 2024 Dec;151:105456. doi: 10.1016/j.jdent.2024.105456. Epub 2024 Nov 9. J Dent. 2024. PMID: 39528154
-
A novel dual-action antimicrobial peptide for caries management.J Dent. 2021 Aug;111:103729. doi: 10.1016/j.jdent.2021.103729. Epub 2021 Jun 17. J Dent. 2021. PMID: 34146653
-
Antibacterial Properties of the Antimicrobial Peptide Gallic Acid-Polyphemusin I (GAPI).Antibiotics (Basel). 2023 Aug 22;12(9):1350. doi: 10.3390/antibiotics12091350. Antibiotics (Basel). 2023. PMID: 37760647 Free PMC article.
-
Efficacy of the dual-action GA-KR12 peptide for remineralising initial enamel caries: an in vitro study.Clin Oral Investig. 2022 Mar;26(3):2441-2451. doi: 10.1007/s00784-021-04210-1. Epub 2021 Oct 12. Clin Oral Investig. 2022. PMID: 34635946
-
A tooth-binding antimicrobial peptide to prevent the formation of dental biofilm.J Mater Sci Mater Med. 2019 Mar 30;30(4):45. doi: 10.1007/s10856-019-6246-6. J Mater Sci Mater Med. 2019. PMID: 30929087
Cited by
-
Stapled Peptides: An Innovative and Ultimate Future Drug Offering a Highly Powerful and Potent Therapeutic Alternative.Biomimetics (Basel). 2024 Sep 5;9(9):537. doi: 10.3390/biomimetics9090537. Biomimetics (Basel). 2024. PMID: 39329559 Free PMC article.
-
Antimicrobial Peptides as Next-Generation Disinfectants: Tackling Biocide and Antimicrobial Resistance in Hospital Hygiene - A Narrative Review.Probiotics Antimicrob Proteins. 2025 Aug 20. doi: 10.1007/s12602-025-10722-z. Online ahead of print. Probiotics Antimicrob Proteins. 2025. PMID: 40833722 Review.
-
In Vitro Investigation of Novel Peptide Hydrogels for Enamel Remineralization.Gels. 2024 Dec 27;11(1):11. doi: 10.3390/gels11010011. Gels. 2024. PMID: 39851984 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

