The Freeze-Drying of Pharmaceuticals in Vials Nested in a Rack System-Part II: Primary Drying Behaviour
- PMID: 38004549
- PMCID: PMC10674193
- DOI: 10.3390/pharmaceutics15112570
The Freeze-Drying of Pharmaceuticals in Vials Nested in a Rack System-Part II: Primary Drying Behaviour
Abstract
The freeze-drying of biopharmaceuticals is a common strategy to extend their shelf-life and facilitate the distribution of therapeutics. The drying phase is the most demanding one in terms of energy consumption and determines the overall process time. Our previous work showed how the loading configuration can impact freezing. This paper focuses on primary drying by comparing the thermal behaviour of vials loaded in direct contact with the shelf or nested in a rack system. The overall heat transfer coefficient from the apparatus to the product was evaluated at different chamber pressures (5-30 Pa) and shelf temperatures (from -10 °C to +30 °C), and in the case of various vial positions (central, semi-border, and border vials). Because of the suspended configuration, the heat transfer coefficient was less affected by chamber pressure in vials nested in a rack system. The two loading configurations displayed comparable heat transfer efficiency below 10 Pa. For higher chamber pressure, the heat transfer coefficients of nested vials were lower than those of vials in direct contact with the shelf. Nevertheless, the rack system was beneficial for reducing the inter-vial variability as it promoted higher uniformity in the heat transfer coefficients of central vials. Eventually, thermal image analyses highlighted limited temperature differences between the vials and the rack system.
Keywords: freeze-drying; heat transfer; mannitol; primary drying; rack system; sublimation flux; sucrose.
Conflict of interest statement
Roberto Pisano, Antonello A. Barresi and Davide Fissore have received funding from Stevanato Group. Maria Chiara Frare is an employee of Stevanato Group. All the authors declare no conflict of interest.
Figures


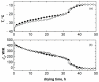
 ) semi-border, and (☐) border vials in direct contact with the shelf. Data reported in (a) and (c) refer to TS = −10 °C for various chamber pressures; data reported in (b) and (d) refer to TS = +10 °C for PC = 10 Pa and TS = +30 °C for Pc = 30 Pa.
) semi-border, and (☐) border vials in direct contact with the shelf. Data reported in (a) and (c) refer to TS = −10 °C for various chamber pressures; data reported in (b) and (d) refer to TS = +10 °C for PC = 10 Pa and TS = +30 °C for Pc = 30 Pa.
 ) semi-border, and (☐) border vials nested in the rack system. Data reported in (a) and (c) refer to TS = −10 °C for various chamber pressures; data reported in (b) and (d) refer to TS = +10 °C for PC = 10 Pa and TS = +30 °C for PC = 30 Pa.
) semi-border, and (☐) border vials nested in the rack system. Data reported in (a) and (c) refer to TS = −10 °C for various chamber pressures; data reported in (b) and (d) refer to TS = +10 °C for PC = 10 Pa and TS = +30 °C for PC = 30 Pa.



References
Grants and funding
LinkOut - more resources
Full Text Sources

