Exploiting Benefits of Vaterite Metastability to Design Degradable Systems for Biomedical Applications
- PMID: 38004553
- PMCID: PMC10674703
- DOI: 10.3390/pharmaceutics15112574
Exploiting Benefits of Vaterite Metastability to Design Degradable Systems for Biomedical Applications
Abstract
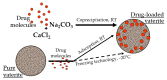
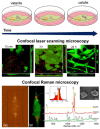
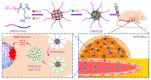
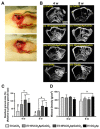
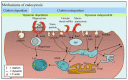
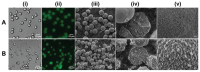
The widespread application of calcium carbonate is determined by its high availability in nature and simplicity of synthesis in laboratory conditions. Moreover, calcium carbonate possesses highly attractive physicochemical properties that make it suitable for a wide range of biomedical applications. This review provides a conclusive analysis of the results on using the tunable vaterite metastability in the development of biodegradable drug delivery systems and therapeutic vehicles with a controlled and sustained release of the incorporated cargo. This manuscript highlights the nuances of vaterite recrystallization to non-porous calcite, dissolution at acidic pH, biodegradation at in vivo conditions and control over these processes. This review outlines the main benefits of vaterite instability for the controlled liberation of the encapsulated molecules for the development of biodegradable natural and synthetic polymeric materials for biomedical purposes.
Keywords: CaCO3 particles; US imaging; anticancer therapy; antimicrobial therapy; ayer-by-layer assembly; biodegradation; buffering; calcite; calcium carbonate; calcium ions; carbon dioxide bubbles; cavitation; controlled release; degradation; dissolution; metastability; ossification; pH-sensitivity; recrystallization; resorption; theranostics; vaterite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures














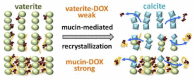

Similar articles
-
Composite Magnetite and Protein Containing CaCO3 Crystals. External Manipulation and Vaterite → Calcite Recrystallization-Mediated Release Performance.ACS Appl Mater Interfaces. 2015 Sep 30;7(38):21315-25. doi: 10.1021/acsami.5b05848. Epub 2015 Sep 15. ACS Appl Mater Interfaces. 2015. PMID: 26348458
-
Calcium carbonate vaterite particles for drug delivery: Advances and challenges.Mater Today Adv. 2022 Jun;14:100214. doi: 10.1016/j.mtadv.2022.100214. Epub 2022 Feb 12. Mater Today Adv. 2022. PMID: 36785703 Free PMC article. Review.
-
Biocompatible superparamagnetic sub-micron vaterite particles for thermo-chemotherapy: From controlled design to in vitro anticancer synergism.Mater Sci Eng C Mater Biol Appl. 2020 Jan;106:110226. doi: 10.1016/j.msec.2019.110226. Epub 2019 Oct 12. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 31753366
-
Difference in cadmium chemisorption on calcite and vaterite porous particles.Chemosphere. 2022 Jun;297:134057. doi: 10.1016/j.chemosphere.2022.134057. Epub 2022 Feb 25. Chemosphere. 2022. PMID: 35227751
-
CaCO₃ vaterite microparticles for biomedical and personal care applications.Mater Sci Eng C Mater Biol Appl. 2014 Dec;45:644-58. doi: 10.1016/j.msec.2014.04.050. Epub 2014 May 14. Mater Sci Eng C Mater Biol Appl. 2014. PMID: 25491874 Review.
Cited by
-
Influence of natural polysaccharides on the morphology and properties of hybrid vaterite microcrystals.Heliyon. 2024 Jun 28;10(13):e33801. doi: 10.1016/j.heliyon.2024.e33801. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39027545 Free PMC article.
References
-
- Liendo F., Arduino M., Deorsola F.A., Bensaid S. Factors Controlling and Influencing Polymorphism, Morphology and Size of Calcium Carbonate Synthesized through the Carbonation Route: A Review. Powder Technol. 2022;398:117050. doi: 10.1016/j.powtec.2021.117050. - DOI
-
- Boyjoo Y., Pareek V.K., Liu J. Synthesis of Micro and Nano-Sized Calcium Carbonate Particles and Their Applications. J. Mater. Chem. A. 2014;2:14270–14288. doi: 10.1039/C4TA02070G. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

