In Vivo Evaluation of a Gastro-Resistant Enprotect® Capsule under Postprandial Conditions
- PMID: 38004555
- PMCID: PMC10674880
- DOI: 10.3390/pharmaceutics15112576
In Vivo Evaluation of a Gastro-Resistant Enprotect® Capsule under Postprandial Conditions
Abstract
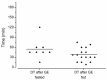
Ready-to-fill enteric hard capsule shells are an evolving field of oral drug and nutraceutical products. Lonza Capsugel® Enprotect® capsules were recently proven to provide reliable release in the small intestine after fasted intake, but robustness against postprandial intake needed to be proven. In this study, the capsules were administered to 16 healthy young subjects after intake of a light meal. The Enprotect® capsules were labelled with 5 mg black iron oxide and 25 mg 13C3-caffeine. Magnetic Resonance Imaging was used to identify the localization and visual dispersion of the capsule filling. The salivary appearance of caffeine was considered a second independent and sensitive marker for the initial release. Whereas the fasted gastric residence time of the capsules amounted to 43 ± 32 min, it was increased to 158 ± 36 min after postprandial intake. Therefore, the mean dispersion time according to MRI and the mean caffeine appearance time were increased to 196 ± 37 min and 189 ± 37 min, respectively. But, similar to fasted administration, no capsule disintegration or leakage was observed in the stomach and 38% of the capsules disintegrated in the jejunum and 62% in the ileum. The mean dispersion time after gastric emptying and the mean caffeine appearance time after gastric emptying amounted to 38 ± 21 min and 31 ± 17 min, respectively. Both did not relevantly change compared to the fasted intake. Only the absolute dispersion time and caffeine appearance were prolonged due to the increased gastric residence and no relevant influence of the light meal was observed on the disintegration or release behavior of Enprotect® capsules after gastric emptying. The capsules also showed robust enteric properties after postprandial administration.
Keywords: MRI; caffeine; enteric hard capsule; fed state; hydroxypropyl methyl cellulose; hydroxypropyl methyl cellulose acetate succinate; saliva; stable isotope.
Conflict of interest statement
The authors A.R., F.M., M.-L.K., M.V.T., W.W. and M.G. are only affiliated with the University of Greifswald and declare no conflict of interest. V.J. and C.D. are employees of the funder, which is the producer of the tested capsule shells. The funder influenced the study design and gave final permission for publication. Conduction of the study and interpretation of results were performed independently by the University of Greifswald. Conduction and interpretation of the study were not influenced by the funder.
Figures




Similar articles
-
In Vivo Evaluation of a Gastro-Resistant HPMC-Based "Next Generation Enteric" Capsule.Pharmaceutics. 2022 Sep 21;14(10):1999. doi: 10.3390/pharmaceutics14101999. Pharmaceutics. 2022. PMID: 36297435 Free PMC article.
-
In vivo characterization of enTRinsic™ drug delivery technology capsule after intake in fed state: A cross-validation approach using salivary tracer technique in comparison to MRI.J Control Release. 2019 Nov 10;313:24-32. doi: 10.1016/j.jconrel.2019.10.023. Epub 2019 Oct 15. J Control Release. 2019. PMID: 31626859 Clinical Trial.
-
Combined Application of MRI and the Salivary Tracer Technique to Determine the in Vivo Disintegration Time of Immediate Release Formulation Administered to Healthy, Fasted Subjects.Mol Pharm. 2019 Apr 1;16(4):1782-1786. doi: 10.1021/acs.molpharmaceut.8b01320. Epub 2019 Mar 8. Mol Pharm. 2019. PMID: 30821987
-
Low dose caffeine as a salivary tracer for the determination of gastric water emptying in fed and fasted state: A MRI validation study.Eur J Pharm Biopharm. 2018 Jun;127:443-452. doi: 10.1016/j.ejpb.2018.03.011. Epub 2018 Mar 27. Eur J Pharm Biopharm. 2018. PMID: 29602018
-
Commercially Available Enteric Empty Hard Capsules, Production Technology and Application.Pharmaceuticals (Basel). 2022 Nov 13;15(11):1398. doi: 10.3390/ph15111398. Pharmaceuticals (Basel). 2022. PMID: 36422528 Free PMC article. Review.
Cited by
-
Use of Magnetic Resonance Imaging for Visualization of Oral Dosage Forms in the Human Stomach: A Scoping Review.Mol Pharm. 2024 Apr 1;21(4):1553-1562. doi: 10.1021/acs.molpharmaceut.3c01123. Epub 2024 Mar 5. Mol Pharm. 2024. PMID: 38440796 Free PMC article.
References
-
- Jannin V., Duysburgh C., Gonzalez V., Govaert M., Agisson M., Marzorati M., Madit N. In vitro evaluation of the gastrointestinal delivery of acid-sensitive pancrelipase in a next generation enteric capsule using an exocrine pancreatic insufficiency disease model. Int. J. Pharm. 2023;630:122441. doi: 10.1016/j.ijpharm.2022.122441. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

