Nanotechnology-Based Drug Delivery Systems to Control Bacterial-Biofilm-Associated Lung Infections
- PMID: 38004561
- PMCID: PMC10674810
- DOI: 10.3390/pharmaceutics15112582
Nanotechnology-Based Drug Delivery Systems to Control Bacterial-Biofilm-Associated Lung Infections
Abstract
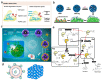
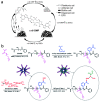
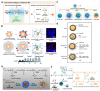
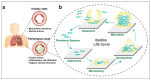
Airway mucus dysfunction and impaired immunological defenses are hallmarks of several lung diseases, including asthma, cystic fibrosis, and chronic obstructive pulmonary diseases, and are mostly causative factors in bacterial-biofilm-associated respiratory tract infections. Bacteria residing within the biofilm architecture pose a complex challenge in clinical settings due to their increased tolerance to currently available antibiotics and host immune responses, resulting in chronic infections with high recalcitrance and high rates of morbidity and mortality. To address these unmet clinical needs, potential anti-biofilm therapeutic strategies are being developed to effectively control bacterial biofilm. This review focuses on recent advances in the development and application of nanoparticulate drug delivery systems for the treatment of biofilm-associated respiratory tract infections, especially addressing the respiratory barriers of concern for biofilm accessibility and the various types of nanoparticles used to combat biofilms. Understanding the obstacles facing pulmonary drug delivery to bacterial biofilms and nanoparticle-based approaches to combatting biofilm may encourage researchers to explore promising treatment modalities for bacterial-biofilm-associated chronic lung infections.
Keywords: bacterial biofilm; biofilm control; chronic lung infections; mucosal barriers; nanoparticle-based drug delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Combination and nanotechnology based pharmaceutical strategies for combating respiratory bacterial biofilm infections.Int J Pharm. 2022 Mar 25;616:121507. doi: 10.1016/j.ijpharm.2022.121507. Epub 2022 Jan 24. Int J Pharm. 2022. PMID: 35085729 Review.
-
Pulmonary biofilm-based chronic infections and inhaled treatment strategies.Int J Pharm. 2021 Jul 15;604:120768. doi: 10.1016/j.ijpharm.2021.120768. Epub 2021 Jun 3. Int J Pharm. 2021. PMID: 34089796 Review.
-
Strategies to Promote the Journey of Nanoparticles Against Biofilm-Associated Infections.Small. 2024 Mar;20(10):e2305988. doi: 10.1002/smll.202305988. Epub 2024 Jan 4. Small. 2024. PMID: 38178276 Review.
-
Advances in pulmonary drug delivery targeting microbial biofilms in respiratory diseases.Nanomedicine (Lond). 2021 Sep;16(21):1905-1923. doi: 10.2217/nnm-2021-0057. Epub 2021 Aug 5. Nanomedicine (Lond). 2021. PMID: 34348474 Review.
-
Nanoparticle-mediated pulmonary drug delivery: state of the art towards efficient treatment of recalcitrant respiratory tract bacterial infections.Drug Deliv Transl Res. 2021 Aug;11(4):1634-1654. doi: 10.1007/s13346-021-00954-1. Epub 2021 Mar 10. Drug Deliv Transl Res. 2021. PMID: 33694082 Free PMC article. Review.
Cited by
-
A cutting-edge new framework for the pain management in children: nanotechnology.Front Mol Neurosci. 2024 Sep 10;17:1391092. doi: 10.3389/fnmol.2024.1391092. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39318422 Free PMC article. Review.
-
Lipid Nanocarriers-Enabled Delivery of Antibiotics and Antimicrobial Adjuvants to Overcome Bacterial Biofilms.Pharmaceutics. 2024 Mar 14;16(3):396. doi: 10.3390/pharmaceutics16030396. Pharmaceutics. 2024. PMID: 38543290 Free PMC article. Review.
-
Biobased Organic Nanoparticles: A Promising Versatile Green Tool for Novel Antimicrobial Agents for Improved Safety.Int J Food Sci. 2025 Jul 18;2025:7955106. doi: 10.1155/ijfo/7955106. eCollection 2025. Int J Food Sci. 2025. PMID: 40718470 Free PMC article. Review.
-
Microbiome Integrity Enhances the Efficacy and Safety of Anticancer Drug.Biomedicines. 2025 Feb 10;13(2):422. doi: 10.3390/biomedicines13020422. Biomedicines. 2025. PMID: 40002835 Free PMC article. Review.
-
Lipid-based nanocarriers for enhanced gentamicin delivery: a comparative study of liquid crystal nanoparticles and liposomes against Escherichia coli biofilms.Drug Deliv Transl Res. 2025 Jun 12. doi: 10.1007/s13346-025-01890-0. Online ahead of print. Drug Deliv Transl Res. 2025. PMID: 40504351
References
-
- Rostami A., Liu M.-C., Xu Q., Li T.-T., Wang T., Jiang B.-G., Lv C.-L., Zhang X.-A., Liu W., Fang L.-Q. Prevalence of human infection with respiratory adenovirus in China: A systematic review and meta-analysis. PLoS Neglected Trop. Dis. 2023;17:e0011151. doi: 10.1371/journal.pntd.0011151. - DOI - PMC - PubMed
Publication types
Grants and funding
- SKLRD-OP-202302/Open Project of State Key Laboratory of Respiratory Disease
- A2023090/Medical Scientific Research Foundation of Guangdong Province
- 2023A1515011064/Guangdong Basic and Applied Basic Research Foundation
- 22qntd4503/Fundamental Research Funds for the Central Universities
- 2019E10020 (KFJJ-202202)/Open Project of The Key Laboratory of Diagnosis and Treatment of Digestive System Tumors of Zhejiang Province
LinkOut - more resources
Full Text Sources

