Cysteamine Eye Drops in Hyaluronic Acid Packaged in Innovative Single-Dose Systems, Part II: Long-Term Stability and Clinical Ocular Biopermanence
- PMID: 38004568
- PMCID: PMC10675239
- DOI: 10.3390/pharmaceutics15112589
Cysteamine Eye Drops in Hyaluronic Acid Packaged in Innovative Single-Dose Systems, Part II: Long-Term Stability and Clinical Ocular Biopermanence
Abstract
Background: Cystinosis is a rare genetic disorder characterized by the accumulation of cystine crystals in several tissues and organs causing, among others, severe eye symptoms. The high instability of cysteamine eye drops makes it difficult to develop formulations with an acceptable shelf life to be prepared in hospital pharmacy departments. Previously, a new compounded formulation of cysteamine eye drops in hyaluronic acid (HA) packaged in innovative single-dose systems was developed.
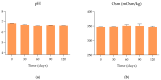
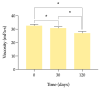
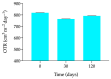
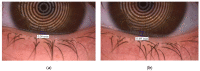
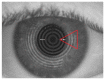
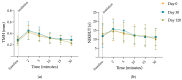
Methods: Long-term stability at -20 °C of this formulation was studied considering the content of cysteamine, pH, osmolality, viscosity, and microbiological analysis. The oxygen permeability of single-dose containers was also studied and an ocular biopermanence study was conducted in healthy volunteers measuring lacrimal stability and volume parameters.
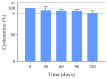
Results: Data confirm that cysteamine concentration remained above 90% for 120 days, all parameters remaining within the accepted range for ophthalmic formulations. The permeability of the containers was reduced over time, while ocular biopermanence was maintained despite the freezing process and storage time.
Conclusions: 0.55% cysteamine hydrochloride formulation in HA and packaged in single-dose containers preserved at -20 °C is stable for 120 days protected from light, presenting high potential for its translation into clinical practice when commercial presentations are not available.
Keywords: cysteamine; cystinosis; eye drops; ophthalmic administration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Cysteamine Eye Drops in Hyaluronic Acid Packaged in Innovative Single-Dose Systems: Stability and Ocular Biopermanence.Pharmaceutics. 2022 Oct 15;14(10):2194. doi: 10.3390/pharmaceutics14102194. Pharmaceutics. 2022. PMID: 36297629 Free PMC article.
-
Limitations and Challenges in the Stability of Cysteamine Eye Drop Compounded Formulations.Pharmaceuticals (Basel). 2021 Dec 21;15(1):2. doi: 10.3390/ph15010002. Pharmaceuticals (Basel). 2021. PMID: 35056058 Free PMC article.
-
Effect of Storage Conditions on Stability of Ophthalmological Compounded Cysteamine Eye Drops.JIMD Rep. 2018;42:47-51. doi: 10.1007/8904_2017_77. Epub 2017 Dec 7. JIMD Rep. 2018. PMID: 29214524 Free PMC article.
-
Recent Research in Ocular Cystinosis: Drug Delivery Systems, Cysteamine Detection Methods and Future Perspectives.Pharmaceutics. 2020 Dec 3;12(12):1177. doi: 10.3390/pharmaceutics12121177. Pharmaceutics. 2020. PMID: 33287176 Free PMC article. Review.
-
Effects of long-term cysteamine treatment in patients with cystinosis.Pediatr Nephrol. 2019 Apr;34(4):571-578. doi: 10.1007/s00467-017-3856-4. Epub 2017 Dec 19. Pediatr Nephrol. 2019. PMID: 29260317 Free PMC article. Review.
Cited by
-
Clinical Effectiveness, Safety, and Compliance of Two Compounded Formulations of Tacrolimus Eye Drops: An Open-Label, Sequential Prospective Study.Int J Mol Sci. 2024 Sep 12;25(18):9847. doi: 10.3390/ijms25189847. Int J Mol Sci. 2024. PMID: 39337336 Free PMC article.
References
-
- Orphanet: Cystinosis. [(accessed on 8 August 2023)]. Available online: https://www.orpha.net/consor/cgi-bin/Disease_Search.php?lng=ES&data_id=1....
-
- Andrzejewska Z., Nevo N., Thomas L., Chhuon C., Bailleux A., Chauvet V., Courtoy P.J., Chol M., Guerrera I.C., Antignac C. Cystinosin Is a Component of the Vacuolar H+-ATPase-Ragulator-Rag Complex Controlling Mammalian Target of Rapamycin Complex 1 Signaling. J. Am. Soc. Nephrol. 2016;27:1678–1688. doi: 10.1681/ASN.2014090937. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

