Targeted Delivery of 5-Fluorouracil and Sonidegib via Surface-Modified ZIF-8 MOFs for Effective Basal Cell Carcinoma Therapy
- PMID: 38004573
- PMCID: PMC10675485
- DOI: 10.3390/pharmaceutics15112594
Targeted Delivery of 5-Fluorouracil and Sonidegib via Surface-Modified ZIF-8 MOFs for Effective Basal Cell Carcinoma Therapy
Abstract
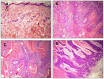
The therapeutic effectiveness of the most widely used anticancer drug 5-fluorouracil (5-FU) is constrained by its high metabolism, short half-life, and rapid drug resistance after chemotherapy. Although various nanodrug delivery systems have been reported for skin cancer therapy, their retention, penetration and targeting are still a matter of concern. Hence, in the current study, a topical gel formulation that contains a metal-organic framework (zeolitic imidazole framework; ZIF-8) loaded with 5-FU and a surface modified with sonidegib (SDG; acting as a therapeutic agent as well as a targeting ligand) (5-FU@ZIF-8 MOFs) is developed against DMBA-UV-induced BCC skin cancer in rats. The MOFs were prepared using one-pot synthesis followed by post drug loading and SDG conjugation. The optimized MOFs were incorporated into hyaluronic acid-hydroxypropyl methyl cellulose gel and further subjected to characterization. Enhanced skin deposition of the 5-FU@ZIF-8-SDG MOFs was observed using ex vivo skin permeation studies. Confocal laser microscopy studies showed that 5-FU@ZIF-8-SDG MOFs permeated the skin via the transfollicular pathway. The 5-FU@ZIF-8-SDG MOFs showed stronger cell growth inhibition in A431 cells and good biocompatibility with HaCaT cells. Histopathological studies showed that the efficacy of the optimized MOF gels improved as the epithelial cells manifested modest hyperplasia, nuclear pleomorphism, and dyskeratosis. Additionally, immunohistochemistry and protein expression studies demonstrated the improved effectiveness of the 5-FU@ZIF-8-SDG MOFs, which displayed a considerable reduction in the expression of Bcl-2 protein. Overall, the developed MOF gels showed good potential for the targeted delivery of multifunctional MOFs in topical formulations for treating BCC cancer.
Keywords: 5-fluorouracil; ZIF-8 MOF; basal cell carcinoma; sonidegib; topical drug delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Gold nanoparticle encapsulated hybrid MOF: synthesis, characterization, and co-drug delivery of 5-fluorouracil and curcumin.Discov Nano. 2024 Dec 11;19(1):201. doi: 10.1186/s11671-024-04152-z. Discov Nano. 2024. PMID: 39661211 Free PMC article.
-
ZIF-8 integrated with polydopamine coating as a novel nano-platform for skin-specific drug delivery.J Mater Chem B. 2023 Feb 22;11(8):1782-1797. doi: 10.1039/d2tb02361j. J Mater Chem B. 2023. PMID: 36727421
-
Fabrication of functional hollow microspheres constructed from MOF shells: Promising drug delivery systems with high loading capacity and targeted transport.Sci Rep. 2016 Nov 23;6:37705. doi: 10.1038/srep37705. Sci Rep. 2016. PMID: 27876876 Free PMC article.
-
Advanced multifunctional nano-lipid carrier loaded gel for targeted delivery of 5-flurouracil and cannabidiol against non-melanoma skin cancer.Environ Res. 2023 Sep 15;233:116454. doi: 10.1016/j.envres.2023.116454. Epub 2023 Jun 19. Environ Res. 2023. PMID: 37343751 Review.
-
Metal-organic frameworks (MOFs) as biomolecules drug delivery systems for anticancer purposes.Eur J Med Chem. 2022 Dec 15;244:114801. doi: 10.1016/j.ejmech.2022.114801. Epub 2022 Oct 4. Eur J Med Chem. 2022. PMID: 36215860 Review.
Cited by
-
Improving the drug delivery performance of ZIF-8 with amine functionalization as a 5-fluorouracil nanocarrier.Sci Rep. 2025 May 29;15(1):18793. doi: 10.1038/s41598-025-03542-2. Sci Rep. 2025. PMID: 40442254 Free PMC article.
-
Biomedical Applications of Metal-Organic Frameworks Revisited.Ind Eng Chem Res. 2025 Jan 14;64(4):1907-1932. doi: 10.1021/acs.iecr.4c03698. eCollection 2025 Jan 29. Ind Eng Chem Res. 2025. PMID: 39906289 Free PMC article. Review.
-
Hybrid Nanoplatforms Based on Photosensitizers and Metal/Covalent Organic Frameworks for Improved Cancer Synergistic Treatment Nano-Delivery Systems.Molecules. 2025 Feb 14;30(4):884. doi: 10.3390/molecules30040884. Molecules. 2025. PMID: 40005193 Free PMC article. Review.
References
-
- Safwat M.A., Soliman G.M., Sayed D., Attia M.A. Fluorouracil-Loaded Gold Nanoparticles for the Treatment of Skin Cancer: Development, In Vitro Characterization, and In Vivo Evaluation in a Mouse Skin Cancer Xenograft Model. Mol. Pharm. 2018;15:2194–2205. doi: 10.1021/acs.molpharmaceut.8b00047. - DOI - PubMed
-
- Chandrashekar N.S., Prasanth V.V. Clinical Evaluation of 5-Fluorouracil from Transdermal Patches on EAC and DLA Cell-Induced Tumors in Mice. Asian Pac. J. Cancer Prev. APJCP. 2008;9:437–440. - PubMed
-
- Alvi I.A., Madan J., Kaushik D., Sardana S., Pandey R.S., Ali A. Comparative Study of Transfersomes, Liposomes, and Niosomes for Topical Delivery of 5-Fluorouracil to Skin Cancer Cells: Preparation, Characterization, in-Vitro Release, and Cytotoxicity Analysis. Anticancer. Drugs. 2011;22:774–782. doi: 10.1097/CAD.0b013e328346c7d6. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

