Oral Drug Absorption and Drug Disposition in Critically Ill Cardiac Patients
- PMID: 38004576
- PMCID: PMC10674156
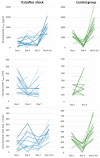
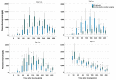
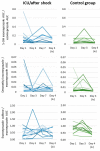
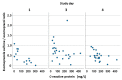
- DOI: 10.3390/pharmaceutics15112598
Oral Drug Absorption and Drug Disposition in Critically Ill Cardiac Patients
Abstract
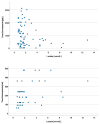
(1) Background: In critically ill cardiac patients, parenteral and enteral food and drug administration routes may be used. However, it is not well known how drug absorption and metabolism are altered in this group of adult patients. Here, we analyze drug absorption and metabolism in patients after cardiogenic shock using the pharmacokinetics of therapeutically indicated esomeprazole. (2) Methods: The pharmacokinetics of esomeprazole were analyzed in a consecutive series of patients with cardiogenic shock and controls before and after elective cardiac surgery. Esomeprazole was administered orally or with a nasogastric tube and once as an intravenous infusion. (3) Results: The maximum plasma concentration and AUC of esomeprazole were, on average, only half in critically ill patients compared with controls (p < 0.005) and remained lower even seven days later. Interestingly, esomeprazole absorption was also markedly compromised on day 1 after elective surgery. The metabolites of esomeprazole showed a high variability between patients. The esomeprazole sulfone/esomeprazole ratio reflecting CYP3A4 activity was significantly lower in critically ill patients even up to day 7, and this ratio was negatively correlated with CRP values (p = 0.002). The 5'-OH-esomeprazole and 5-O-desmethyl-esomeprazol ratios reflecting CYP2C19 activity did not differ significantly between critically ill and control patients. (4) Conclusions: Gastrointestinal drug absorption can be significantly reduced in critically ill cardiac patients compared with elective patients with stable cardiovascular disease. The decrease in bioavailability indicates that, under these conditions, any vital medication should be administered intravenously to maintain high levels of medications. After shock, hepatic metabolism via the CYP3A4 enzyme may be reduced.
Keywords: cardiogenic shock; enteral medication; esomeprazole; intensive care medication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Elke G., van Zanten A.R., Lemieux M., McCall M., Jeejeebhoy K.N., Kott M., Jiang X., Day A.G., Heyland D.K. Enteral versus parenteral nutrition in critically ill patients: An updated systematic review and meta-analysis of randomized controlled trials. Crit. Care. 2016;20:117. doi: 10.1186/s13054-016-1298-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

