Delivery Strategies of Probiotics from Nano- and Microparticles: Trends in the Treatment of Inflammatory Bowel Disease-An Overview
- PMID: 38004578
- PMCID: PMC10674632
- DOI: 10.3390/pharmaceutics15112600
Delivery Strategies of Probiotics from Nano- and Microparticles: Trends in the Treatment of Inflammatory Bowel Disease-An Overview
Abstract
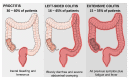
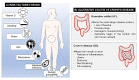
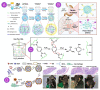
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder, most known as ulcerative colitis (UC) and Crohn's disease (CD), that affects the gastrointestinal tract (GIT), causing considerable symptoms to millions of people around the world. Conventional therapeutic strategies have limitations and side effects, prompting the exploration of innovative approaches. Probiotics, known for their potential to restore gut homeostasis, have emerged as promising candidates for IBD management. Probiotics have been shown to minimize disease symptoms, particularly in patients affected by UC, opening important opportunities to better treat this disease. However, they exhibit limitations in terms of stability and targeted delivery. As several studies demonstrate, the encapsulation of the probiotics, as well as the synthetic drug, into micro- and nanoparticles of organic materials offers great potential to solve this problem. They resist the harsh conditions of the upper GIT portions and, thus, protect the probiotic and drug inside, allowing for the delivery of adequate amounts directly into the colon. An overview of UC and CD, the benefits of the use of probiotics, and the potential of micro- and nanoencapsulation technologies to improve IBD treatment are presented. This review sheds light on the remarkable potential of nano- and microparticles loaded with probiotics as a novel and efficient strategy for managing IBD. Nonetheless, further investigations and clinical trials are warranted to validate their long-term safety and efficacy, paving the way for a new era in IBD therapeutics.
Keywords: drug delivery systems; gastrointestinal tract; inflammatory bowel disease; microparticles; nanoparticles; probiotics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Inflammatory Bowel Disease: The Emergence of New Trends in Lifestyle and Nanomedicine as the Modern Tool for Pharmacotherapy.Nanomaterials (Basel). 2020 Dec 9;10(12):2460. doi: 10.3390/nano10122460. Nanomaterials (Basel). 2020. PMID: 33316984 Free PMC article. Review.
-
The Effectiveness of Probiotics in the Treatment of Inflammatory Bowel Disease (IBD)-A Critical Review.Nutrients. 2020 Jul 2;12(7):1973. doi: 10.3390/nu12071973. Nutrients. 2020. PMID: 32630805 Free PMC article. Review.
-
Probiotics for inflammatory bowel disease: Is there sufficient evidence?Open Life Sci. 2024 Apr 5;19(1):20220821. doi: 10.1515/biol-2022-0821. eCollection 2024. Open Life Sci. 2024. PMID: 38585636 Free PMC article. Review.
-
Probiotics for inflammatory bowel diseases: a promising adjuvant treatment.Int J Food Sci Nutr. 2019 Feb;70(1):20-29. doi: 10.1080/09637486.2018.1477123. Epub 2018 May 28. Int J Food Sci Nutr. 2019. PMID: 29804478 Review.
-
The single-cell modification strategies for probiotics delivery in inflammatory bowel disease: A review.Carbohydr Polym. 2024 Jan 15;324:121472. doi: 10.1016/j.carbpol.2023.121472. Epub 2023 Oct 9. Carbohydr Polym. 2024. PMID: 37985038 Review.
Cited by
-
Analysis of nanomedicine applications for inflammatory bowel disease: structural and temporal dynamics, research hotspots, and emerging trends.Front Pharmacol. 2025 Jan 8;15:1523052. doi: 10.3389/fphar.2024.1523052. eCollection 2024. Front Pharmacol. 2025. PMID: 39845796 Free PMC article.
-
Advances in bio-polymer coatings for probiotic microencapsulation: chitosan and beyond for enhanced stability and controlled release.Des Monomers Polym. 2024 Dec 31;28(1):1-34. doi: 10.1080/15685551.2024.2448122. eCollection 2025. Des Monomers Polym. 2024. PMID: 39777298 Free PMC article. Review.
-
Probiotics in Nanotechnology-Driven Wound Healing: From Mechanistic Insight to Clinical Promise.Pharmaceutics. 2025 Jun 21;17(7):805. doi: 10.3390/pharmaceutics17070805. Pharmaceutics. 2025. PMID: 40733015 Free PMC article. Review.
-
Recognizing the biological barriers and pathophysiological characteristics of the gastrointestinal tract for the design and application of nanotherapeutics.Drug Deliv. 2024 Dec;31(1):2415580. doi: 10.1080/10717544.2024.2415580. Epub 2024 Oct 15. Drug Deliv. 2024. PMID: 39404464 Free PMC article. Review.
-
Insights into the unique roles of extracellular vesicles for gut health modulation: Mechanisms, challenges, and perspectives.Curr Res Microb Sci. 2024 Oct 21;7:100301. doi: 10.1016/j.crmicr.2024.100301. eCollection 2024. Curr Res Microb Sci. 2024. PMID: 39525958 Free PMC article. Review.
References
-
- Molodecky N.A., Soon I.S., Rabi D.M., Ghali W.A., Ferris M., Chernoff G., Benchimol E.I., Panaccione R., Ghosh S., Barkema H.W., et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases with Time, Based on Systematic Review. Gastroenterology. 2012;142:46–54.e42. doi: 10.1053/j.gastro.2011.10.001. - DOI - PubMed
-
- Brito R.C.V., Peres De C.L., Silveira K.A.F., Arruda E.L., de Almeida Júnior M.P. Doenças Inflamatórias Intestinais No Brasil: Perfil Das Internações, Entre Os Anos de 2009 a 2019. Rev. Educ. Saúde. 2020;8:127–135. doi: 10.29237/2358-9868.2020v8i1.p127-135. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

