Therapeutic Activity of a Topical Formulation Containing 8-Hydroxyquinoline for Cutaneous Leishmaniasis
- PMID: 38004580
- PMCID: PMC10675550
- DOI: 10.3390/pharmaceutics15112602
Therapeutic Activity of a Topical Formulation Containing 8-Hydroxyquinoline for Cutaneous Leishmaniasis
Abstract
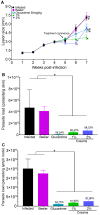
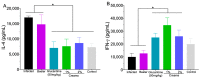
Cutaneous leishmaniasis exhibits a wide spectrum of clinical manifestations; however, only a limited number of drugs are available and include Glucantime® and amphotericin B, which induce unacceptable side effects in patients, limiting their use. Thus, there is an urgent demand to develop a treatment for leishmaniasis. Recently, it was demonstrated that 8-hydroxyquinoline (8-HQ) showed significant leishmanicidal effects in vitro and in vivo. Based on that, this work aimed to develop a topical formulation containing 8-HQ and assess its activity in experimental cutaneous leishmaniasis. 8-HQ was formulated using a Beeler base at 1 and 2% and showed an emulsion size with a D50 of 25 and 51.3 µm, respectively, with a shear-thinning rheological behaviour. The creams were able to permeate artificial Strat-M membranes and excised porcine skin without causing any morphological changes in the porcine skin or murine skin tested. In BALB/c mice infected with L. (L.) amazonensis, topical treatment with creams containing 1 or 2% of 8-HQ was found to reduce the parasite burden and lesion size compared to infected controls with comparable efficacy to Glucantime® (50 mg/kg) administered at the site of the cutaneous lesion. In the histological section of the skin from infected controls, a diffuse inflammatory infiltrate with many heavily infected macrophages that were associated with areas of necrosis was observed. On the other hand, animals treated with both creams showed only moderate inflammatory infiltrate, characterised by few infected macrophages, while tissue necrosis was not observed. These histological characteristics in topically treated animals were associated with an increase in the amount of IFN-γ and a reduction in IL-4 levels. The topical use of 8-HQ was active in decreasing tissue parasitism and should therefore be considered an interesting alternative directed to the treatment of leishmaniasis, considering that this type of treatment is non-invasive, painless, and, importantly, does not require hospitalisation, improving patient compliance by allowing the treatment to be conducted.
Keywords: 8-hydroxyquinoline; L. (L.) amazonensis; cutaneous leishmaniasis; quinolines; topical treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
High Selectivity of 8-Hydroxyquinoline on Leishmania (Leishmania) and Leishmania (Viannia) Species Correlates with a Potent Therapeutic Activity In Vivo.Pharmaceuticals (Basel). 2023 May 7;16(5):707. doi: 10.3390/ph16050707. Pharmaceuticals (Basel). 2023. PMID: 37242490 Free PMC article.
-
An 8-hydroxyquinoline-containing polymeric micelle system is effective for the treatment of murine tegumentary leishmaniasis.Parasitol Res. 2016 Nov;115(11):4083-4095. doi: 10.1007/s00436-016-5181-4. Epub 2016 Jul 1. Parasitol Res. 2016. PMID: 27365053
-
Drug-containing hydrophobic dressings as a topical experimental therapy for cutaneous leishmaniasis.J Parasit Dis. 2020 Mar;44(1):79-87. doi: 10.1007/s12639-019-01162-y. Epub 2019 Sep 20. J Parasit Dis. 2020. PMID: 32174708 Free PMC article.
-
Topical buparvaquone nano-enabled hydrogels for cutaneous leishmaniasis.Int J Pharm. 2020 Oct 15;588:119734. doi: 10.1016/j.ijpharm.2020.119734. Epub 2020 Aug 7. Int J Pharm. 2020. PMID: 32777535
-
Pharmacokinetics and pharmacodynamics in the treatment of cutaneous leishmaniasis - challenges and opportunities.RSC Med Chem. 2021 Jan 7;12(4):472-482. doi: 10.1039/d0md00343c. RSC Med Chem. 2021. PMID: 34041488 Free PMC article. Review.
Cited by
-
Dicentrine Purified from the Leaves of Ocotea puberula Controls the Intracellular Spread of L. (L.) amazonensis and L. (V.) braziliensis Amastigotes and Has Therapeutic Activity as a Topical Treatment in Experimental Cutaneous Leishmaniasis.Microorganisms. 2025 Jan 30;13(2):309. doi: 10.3390/microorganisms13020309. Microorganisms. 2025. PMID: 40005676 Free PMC article.
References
-
- Jain S., Chandra V., Kumar Jain P., Pathak K., Pathak D., Vaidya A. Comprehensive Review on Current Developments of Quinoline-Based Anticancer Agents. Arab. J. Chem. 2019;12:4920–4946. doi: 10.1016/j.arabjc.2016.10.009. - DOI
-
- Albrecht M., Fiege M., Osetska O. 8-Hydroxyquinolines in Metallosupramolecular Chemistry. Coord. Chem. Rev. 2008;252:812–824. doi: 10.1016/j.ccr.2007.06.003. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

