Targeting Glucose Metabolism in Cancer Cells as an Approach to Overcoming Drug Resistance
- PMID: 38004589
- PMCID: PMC10675572
- DOI: 10.3390/pharmaceutics15112610
Targeting Glucose Metabolism in Cancer Cells as an Approach to Overcoming Drug Resistance
Abstract
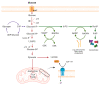
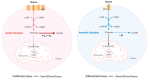
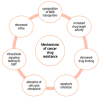
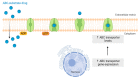
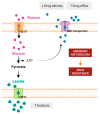
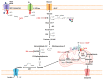
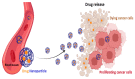
The "Warburg effect" consists of a metabolic shift in energy production from oxidative phosphorylation to glycolysis. The continuous activation of glycolysis in cancer cells causes rapid energy production and an increase in lactate, leading to the acidification of the tumour microenvironment, chemo- and radioresistance, as well as poor patient survival. Nevertheless, the mitochondrial metabolism can be also involved in aggressive cancer characteristics. The metabolic differences between cancer and normal tissues can be considered the Achilles heel of cancer, offering a strategy for new therapies. One of the main causes of treatment resistance consists of the increased expression of efflux pumps, and multidrug resistance (MDR) proteins, which are able to export chemotherapeutics out of the cell. Cells expressing MDR proteins require ATP to mediate the efflux of their drug substrates. Thus, inhibition of the main energy-producing pathways in cancer cells, not only induces cancer cell death per se, but also overcomes multidrug resistance. Given that most anticancer drugs do not have the ability to distinguish normal cells from cancer cells, a number of drug delivery systems have been developed. These nanodrug delivery systems provide flexible and effective methods to overcome MDR by facilitating cellular uptake, increasing drug accumulation, reducing drug efflux, improving targeted drug delivery, co-administering synergistic agents, and increasing the half-life of drugs in circulation.
Keywords: Warburg effect; glycolysis; nanoparticles; resistance; tumor metabolism; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Nanodrug delivery in reversing multidrug resistance in cancer cells.Front Pharmacol. 2014 Jul 10;5:159. doi: 10.3389/fphar.2014.00159. eCollection 2014. Front Pharmacol. 2014. PMID: 25071577 Free PMC article. Review.
-
Improvement of conventional anti-cancer drugs as new tools against multidrug resistant tumors.Drug Resist Updat. 2020 May;50:100682. doi: 10.1016/j.drup.2020.100682. Epub 2020 Feb 7. Drug Resist Updat. 2020. PMID: 32087558
-
Revisiting the Warburg effect: historical dogma versus current understanding.J Physiol. 2021 Mar;599(6):1745-1757. doi: 10.1113/JP278810. Epub 2021 Jan 4. J Physiol. 2021. PMID: 33347611 Review.
-
A targeted nanoplatform co-delivering chemotherapeutic and antiangiogenic drugs as a tool to reverse multidrug resistance in breast cancer.Acta Biomater. 2018 Jul 15;75:398-412. doi: 10.1016/j.actbio.2018.05.050. Epub 2018 Jun 3. Acta Biomater. 2018. PMID: 29874597
-
Tumor microenvironment and epithelial mesenchymal transition as targets to overcome tumor multidrug resistance.Drug Resist Updat. 2020 Dec;53:100715. doi: 10.1016/j.drup.2020.100715. Epub 2020 Jun 20. Drug Resist Updat. 2020. PMID: 32679188 Review.
Cited by
-
Mitochondrial inhibitors: a new horizon in breast cancer therapy.Front Pharmacol. 2024 Jul 4;15:1421905. doi: 10.3389/fphar.2024.1421905. eCollection 2024. Front Pharmacol. 2024. PMID: 39027328 Free PMC article. Review.
-
Evolving Treatment Landscape of Frontline Therapy for Metastatic Urothelial Carcinoma: Current Insights and Future Perspectives.Cancers (Basel). 2024 Dec 5;16(23):4078. doi: 10.3390/cancers16234078. Cancers (Basel). 2024. PMID: 39682263 Free PMC article. Review.
-
Intrinsically synthesized melatonin in mitochondria and factors controlling its production.Histol Histopathol. 2025 Mar;40(3):271-282. doi: 10.14670/HH-18-776. Epub 2024 Jun 7. Histol Histopathol. 2025. PMID: 38920277 Review.
-
An in- vitro measurement for the toxicity of peptides inhibit hexokinase II in breast cancer cell lines.Sci Rep. 2025 Mar 27;15(1):10660. doi: 10.1038/s41598-025-94858-6. Sci Rep. 2025. PMID: 40148397 Free PMC article.
-
Non-coding RNAs: emerging biomarkers and therapeutic targets in cancer and inflammatory diseases.Front Oncol. 2025 Mar 10;15:1534862. doi: 10.3389/fonc.2025.1534862. eCollection 2025. Front Oncol. 2025. PMID: 40129920 Free PMC article. Review.
References
Publication types
Grants and funding
- MetabRes_CESPU_2017CESPU/Cooperativa de Ensino Superior Politécnico e Universitário
- Flav4Tumor_GI2-CESPU_2022/Cooperativa de Ensino Superior Politécnico e Universitário
- Norte-01-0145-FEDER-000051 - "Cancer Research on Therapy Resistance: From Basic Mecha-nisms to Novel Targets"/European Regional Development Fund (FEDER)
LinkOut - more resources
Full Text Sources

