Aerogels as Carriers for Oral Administration of Drugs: An Approach towards Colonic Delivery
- PMID: 38004617
- PMCID: PMC10674668
- DOI: 10.3390/pharmaceutics15112639
Aerogels as Carriers for Oral Administration of Drugs: An Approach towards Colonic Delivery
Abstract
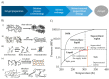
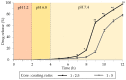
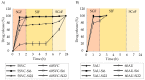
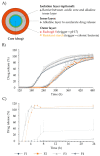
Polysaccharide aerogels have emerged as a highly promising technology in the field of oral drug delivery. These nanoporous, ultralight materials, derived from natural polysaccharides such as cellulose, starch, or chitin, have significant potential in colonic drug delivery due to their unique properties. The particular degradability of polysaccharide-based materials by the colonic microbiota makes them attractive to produce systems to load, protect, and release drugs in a controlled manner, with the capability to precisely target the colon. This would allow the local treatment of gastrointestinal pathologies such as colon cancer or inflammatory bowel diseases. Despite their great potential, these applications of polysaccharide aerogels have not been widely explored. This review aims to consolidate the available knowledge on the use of polysaccharides for oral drug delivery and their performance, the production methods for polysaccharide-based aerogels, the drug loading possibilities, and the capacity of these nanostructured systems to target colonic regions.
Keywords: aerogels; colonic drug delivery; inflammatory bowel diseases; oral administration; polysaccharides; porous systems.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

