Unusual Oligomeric Laccase-like Oxidases from Ascomycete Curvularia geniculata VKM F-3561 Polymerizing Phenylpropanoids and Phenolic Compounds under Neutral Environmental Conditions
- PMID: 38004710
- PMCID: PMC10673308
- DOI: 10.3390/microorganisms11112698
Unusual Oligomeric Laccase-like Oxidases from Ascomycete Curvularia geniculata VKM F-3561 Polymerizing Phenylpropanoids and Phenolic Compounds under Neutral Environmental Conditions
Abstract
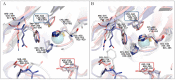
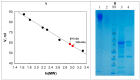
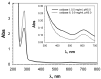
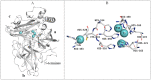
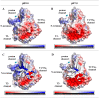
The unique oligomeric alkaliphilic laccase-like oxidases of the ascomycete C. geniculata VKM F-3561 (with molecular masses about 1035 and 870 kDa) were purified and characterized for the first time. The ability of the enzymes to oxidize phenylpropanoids and phenolic compounds under neutral environmental conditions with the formation of previously unknown di-, tri-, and tetrameric products of transformation was shown. The possibility to obtain industrially valuable compounds (dihydroxybenzyl alcohol and hydroxytyrosol) from caffeic acid using laccase-like oxidases of C. geniculata VKM F-3561 has been shown. Complete nucleotide sequence of the laccase gene, which is expressed at the peak of alkaliphilic laccase activity of the fungus, and its promoter region were determined. Based on the phylogenetic analysis of the nucleotide sequence, the nearest relationship of the isolated laccase gene with similar genes of fungi of the genera Alternaria, Bipolaris, and Cochliobolus was shown. Homologous model of the laccase structure was predicted and a proton channel was found, which was presumably responsible for the accumulation and transport of protons to T2/T3-copper center in the alkaliphilic laccase molecule and providing the functional activity of the enzyme in the neutral alkaline environment conditions.
Keywords: alkaliphilic laccase; ascomycete; laccase-like oxidase; neutral environmental conditions; phenolic compounds; phenylpropanoids; polymerization; proton channel; transformation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




Similar articles
-
The Laccase of Myrothecium roridum VKM F-3565: A New Look at Fungal Laccase Tolerance to Neutral and Alkaline Conditions.Chembiochem. 2023 Feb 14;24(4):e202200600. doi: 10.1002/cbic.202200600. Epub 2023 Jan 18. Chembiochem. 2023. PMID: 36513608
-
Structure-function studies of a novel laccase-like multicopper oxidase from Thermothelomyces thermophila provide insights into its biological role.Acta Crystallogr D Struct Biol. 2023 Jul 1;79(Pt 7):641-654. doi: 10.1107/S2059798323004175. Epub 2023 Jun 16. Acta Crystallogr D Struct Biol. 2023. PMID: 37326583 Free PMC article.
-
Identification and characterization of laccase-type multicopper oxidases involved in dye-decolorization by the fungus Leptosphaerulina sp.BMC Biotechnol. 2015 Aug 14;15:74. doi: 10.1186/s12896-015-0192-2. BMC Biotechnol. 2015. PMID: 26268358 Free PMC article.
-
Mode of Action, Properties, Production, and Application of Laccase: A Review.Recent Pat Biotechnol. 2019;13(1):19-32. doi: 10.2174/1872208312666180821161015. Recent Pat Biotechnol. 2019. PMID: 30147019 Review.
-
The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin. Modelling and structural relationships.Eur J Biochem. 1990 Jan 26;187(2):341-52. doi: 10.1111/j.1432-1033.1990.tb15311.x. Eur J Biochem. 1990. PMID: 2404764 Review.
Cited by
-
Enzymatic Treatment of Lignin in Alkaline Homogeneous Systems: A Review on Alkaliphilic Laccases.ChemSusChem. 2025 May 19;18(10):e202402377. doi: 10.1002/cssc.202402377. Epub 2025 Jan 28. ChemSusChem. 2025. PMID: 39815958 Free PMC article. Review.
References
-
- Ferry Y., Leech D. Amperometric detection of catecholamine neurotransmitters using electrocatalytic substrate recycling at a laccase electrode. Electroanalysis. 2005;17:113–119. doi: 10.1002/elan.200403069. - DOI
-
- Szczupak A., Halámek J., Halámková L., Bocharova V., Alfonta L., Katz E. Living battery—Biofuel cells operating in vivo in clams. Energy Environ. Sci. 2012;5:8891–8895. doi: 10.1039/c2ee21626d. - DOI
-
- Reuillard B., Abreu C., Lalaoui N., Le Goff A., Holzinger M., Ondel O., Buret F., Cosnier S. One-year stability for a glucose/oxygen biofuel cell combined with pH reactivation of the laccase/carbon nanotube biocathode. Bioelectrochemistry. 2015;106:73–77. doi: 10.1016/j.bioelechem.2015.04.009. - DOI - PubMed
-
- Vicente A.I., Viña-Gonzalez J., Santos-Moriano P., Marquez-Alvarez C., Ballesteros A.O., Alcalde M. Evolved alkaline fungal laccase secreted by Saccharomyces cerevisiae as useful tool for the synthesis of C–N heteropolymeric dye. J. Mol. Catal. B Enzym. 2016;134:323–330. doi: 10.1016/j.molcatb.2016.10.004. - DOI
LinkOut - more resources
Full Text Sources

