Impacts of Oak Mulch Amendments on Rhizosphere Microbiome of Citrus Trees Grown in Florida Flatwood Soils
- PMID: 38004775
- PMCID: PMC10673100
- DOI: 10.3390/microorganisms11112764
Impacts of Oak Mulch Amendments on Rhizosphere Microbiome of Citrus Trees Grown in Florida Flatwood Soils
Abstract
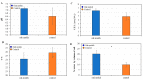
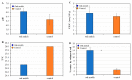
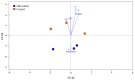
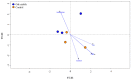
Rhizosphere interactions are an understudied component of citrus production. This is even more important in Florida flatwood soils, which pose significant challenges in achieving sustainable and effective fruit production due to low natural fertility and organic matter. Citrus growers apply soil amendments, including oak mulch, to ameliorate their soil conditions. Thus, the aim of this research was to evaluate the effects of oak mulch on citrus nutrient uptake, soil characteristics, and rhizosphere composition. The plant material consisted of 'Valencia' sweet orange (Citrus × sinensis) trees grafted on 'US-812' (C. reticulata × C. trifoliata) rootstock. The experiment consisted of two treatments, which included trees treated with oak mulch (300 kg of mulch per plot) and a control. The soil and leaf nutrient contents, soil pH, cation exchange capacity, moisture, temperature, and rhizosphere bacterial compositions were examined over the course of one year (spring and fall 2021). During the spring samplings, the citrus trees treated with oak mulch resulted in significantly greater soil Zn and Mn contents, greater soil moisture, and greater rhizosphere bacterial diversity compared to the control, while during the fall samplings, only a greater soil moisture content was observed in the treated trees. The soil Zn and Mn content detected during the spring samplings correlated with the significant increases in the diversity of the rhizosphere bacterial community composition. Similarly, the reduced rates of leaching and evaporation (at the soil surface) of oak mulch applied to Florida sandy soils likely played a large role in the significant increase in moisture and nutrient retention.
Keywords: citrus; flatwoods; microbiome; soil amendments; soil nutrient.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Grapefruit Root and Rhizosphere Responses to Varying Planting Densities, Fertilizer Concentrations and Application Methods.Plants (Basel). 2023 Apr 15;12(8):1659. doi: 10.3390/plants12081659. Plants (Basel). 2023. PMID: 37111884 Free PMC article.
-
Changes to the Bacterial Microbiome in the Rhizosphere and Root Endosphere of Persea americana (Avocado) Treated With Organic Mulch and a Silicate-Based Mulch or Phosphite, and Infested With Phytophthora cinnamomi.Front Microbiol. 2022 Apr 28;13:870900. doi: 10.3389/fmicb.2022.870900. eCollection 2022. Front Microbiol. 2022. PMID: 35572652 Free PMC article.
-
Interactions between rootstocks and compost influence the active rhizosphere bacterial communities in citrus.Microbiome. 2023 Apr 20;11(1):79. doi: 10.1186/s40168-023-01524-y. Microbiome. 2023. PMID: 37076924 Free PMC article.
-
Micronutrients Improve Growth and Development of HLB-Affected Citrus Trees in Florida.Plants (Basel). 2022 Dec 23;12(1):73. doi: 10.3390/plants12010073. Plants (Basel). 2022. PMID: 36616206 Free PMC article. Review.
-
Etiology of three recent diseases of citrus in São Paulo State: sudden death, variegated chlorosis and huanglongbing.IUBMB Life. 2007 Apr-May;59(4-5):346-54. doi: 10.1080/15216540701299326. IUBMB Life. 2007. PMID: 17505974 Review.
Cited by
-
Multivariate analysis between environmental factors and fruit quality of citrus at the core navel orange-producing area in China.Front Plant Sci. 2024 Dec 2;15:1510827. doi: 10.3389/fpls.2024.1510827. eCollection 2024. Front Plant Sci. 2024. PMID: 39717729 Free PMC article.
References
-
- Ozores-Hampton M., Stansly P.A., McSorley R., Obreza T.A. Effects of Long-term Organic Amendments and Soil Solarization on Pepper and Watermelon Growth, Yield, and Soil Fertility. HortScience. 2005;40:80–84. doi: 10.21273/HORTSCI.40.1.80. - DOI
-
- Singerman A., Useche P. Impact of Citrus Greening on Citrus Operations in Florida. University of Florida, Institute of Food and Agriculture Sciences, Electronic Data Information Source (UF/IFAS EDIS); Gainesville, FL, USA: 2016. [(accessed on 15 October 2023)]. FE983. Available online: https://edis.ifas.ufl.edu/publication/FE983.
-
- Singerman A., Burani-Arouca M., Futch S.H. The Profitability of New Citrus Plantings in Florida in the Era of Huanglongbing. HortScience. 2018;53:1655–1663. doi: 10.21273/HORTSCI13410-18. - DOI
-
- Zapien-Macias J.M., Ferrarezi R.S., Spyke P.D., Castle W.S., Gmitter F.G., Grosser J.W., Rossi L. Early Performance of Recently Released Rootstocks with Grapefruit, Navel Orange, and Mandarin Scions under Endemic Huanglongbing Conditions in Florida. Horticulturae. 2022;8:1027. doi: 10.3390/horticulturae8111027. - DOI
-
- Achor D., Etxeberria E., Wang N. Citrus affected with huanglongbing disease. Plant Pathol. J. 2010;9:56–64. doi: 10.3923/ppj.2010.56.64. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

