Changing Strategies for the Detection of Bacteria in Platelet Components in Ireland: From Primary and Secondary Culture (2010-2020) to Large Volume Delayed Sampling (2020-2023)
- PMID: 38004776
- PMCID: PMC10673373
- DOI: 10.3390/microorganisms11112765
Changing Strategies for the Detection of Bacteria in Platelet Components in Ireland: From Primary and Secondary Culture (2010-2020) to Large Volume Delayed Sampling (2020-2023)
Abstract
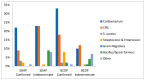
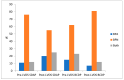
Bacterial contamination of platelet components (PC) poses the greatest microbial risk to recipients, as bacteria can multiply over the course of PC storage at room temperature. Between 2010 and 2020, the Irish Blood Transfusion Service (IBTS) screened over 170,000 buffy coat-derived pooled (BCDP) and single-donor apheresis platelets (SDAPs) with the BACT/ALERT 3D microbial detection system (Biomerieux, L'Etoile, France), using a two-step screening protocol which incorporated primary and secondary cultures. Although the protocol was successful in averting septic transfusion reactions (STRs), testing large sample volumes at later time points was reported to improve detection of bacterial contamination. A modified large-volume delayed sampling (LVDS)-type protocol was adopted in 2020, which in the case of SDAP was applied to collections rather than individual splits (2020-2023, 44,642 PC screened). Rates of bacterial contamination for BCDP were 0.125% on Day-2, 0.043% on Day-4 vs. 0.191% in the post-LVDS period. SDAP contamination rates in the pre-LVDS period were 0.065% on Day-1, 0.017% on Day-4 vs. 0.072% in the post-LVDS period. Confirmed STRs were absent, and the interdiction rate for possibly contaminated SDAP was over 70%. In the post-LVDS period, BCDPs had a higher total positivity rate than SDAPs, 0.191% (1:525) versus 0.072% (1:1385), respectively, (chi-squared 12.124, 1 df, p = 0.0005). The majority of organisms detected were skin-flora-type, low pathogenicity organisms, including coagulase-negative staphylococci and Cutibacterium acnes, with little change in the frequency of clinically significant organisms identified over time. Both protocols prevented the issue of potentially harmful components contaminated (rarely) with a range of pathogenic bacteria, including Escherichia coli, Serratia marcesens, Staphylococcus aureus, and streptococci. Culture positivity of outdates post-LVDS whereby 100% of expired platelets are retested provides a residual risk estimate of 0.06% (95% CI 0.016-0.150). However, bacterial contamination rates in expired platelets did not demonstrate a statistically significant difference between the pre-LVDS 0.100% (CI 0.033-0.234) and post-LVDS 0.059% (0.016-0.150) periods (chi-squared = 0.651, 1 df, p = 0.42).
Keywords: bacteria; bacterial culture; contamination rates; platelet components (PCs); risk; safety; transfusion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Bonnel P.H. Bacterial Contamination of Preserved Blood and Derivatives. Vox Sang. 1961;6:60–67. doi: 10.1111/j.1423-0410.1961.tb03136.x. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous