Therapeutic Effects of Zymomonas mobilis on Experimental DSS-Induced Colitis Mouse Model
- PMID: 38004805
- PMCID: PMC10672878
- DOI: 10.3390/microorganisms11112793
Therapeutic Effects of Zymomonas mobilis on Experimental DSS-Induced Colitis Mouse Model
Abstract
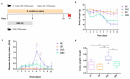
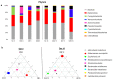
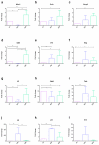
Zymomonas mobilis, a Gram-negative bacteria observed in some popular beverages, is considered safe and has been studied for its potential therapeutic benefits. In this study, we explored its effects on the inflammatory process, tissue integrity, differential gene expression, and microbiota composition in an experimental dextran sulfate sodium (DSS)-induced colitis model in mice. As a result, Z. mobilis alleviated the symptoms caused by DSS administration, as indicated by reduced weight loss, disease activity index, a significant reduction in the colon weight/length ratio, and histopathological improvement. Also, Z. mobilis could restore the mucosal barrier as well as increase the expression of Muc3 and Ocln genes. An analysis of 16S rRNA sequences showed that Z. mobilis alters gut microbiota, increasing Akkermansia muciniphila abundance and decreasing Escherichia coli. Furthermore, Z. mobilis seems to be involved in potentiating a regulatory phenotype by inducing immunomodulatory genes like Tgfb, Il5, Il10, and Foxp3 and reducing the relative mRNA expression of proinflammatory cytokines TNF, Il6, and Il17. Our data suggest that Z. mobilis could alleviate disease progression and be considered a possible probiotic adjuvant for pathologies of the bowel.
Keywords: DSS-induced colitis; Zymomonas mobilis; immunomodulation; microbiota; probiotics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- de Azerêdo G.A., Stamford T.L.M., de Souza E.L., Veras F.F., de Almeida E.R., de Araújo J.M. In Vivo Assessment of Possible Probiotic Properties of Zymomonas Mobilis in a Wistar Rat Model. Electron. J. Biotechnol. 2010;13:3–4. doi: 10.2225/vol13-issue2-fulltext-4. - DOI
-
- Tallyne de Aguiar Silva A., Lima Cavalcanti I.D., Ayanny de Lima Fernandes M., Gisele de Oliveira Coimbra C., Manoella de Souza Lima G. Effect of Zymomonas Mobilis Probiotic on Cholesterol and Its Lipoprotein Fractions and the Intestinal Regulation. Clin. Nutr. 2020;39:3750–3755. doi: 10.1016/j.clnu.2020.04.002. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

