Efficacy, Safety, and Concerns on Microbiota Modulation, Antibiotics, Probiotics, and Fecal Microbial Transplant for Inflammatory Bowel Disease and Other Gastrointestinal Conditions: Results from an International Survey
- PMID: 38004817
- PMCID: PMC10672996
- DOI: 10.3390/microorganisms11112806
Efficacy, Safety, and Concerns on Microbiota Modulation, Antibiotics, Probiotics, and Fecal Microbial Transplant for Inflammatory Bowel Disease and Other Gastrointestinal Conditions: Results from an International Survey
Abstract
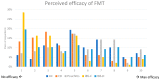
The gut microbiota play a pivotal role in human health. Dysbiosis, alterations in microbiota composition and function, is associated with gastrointestinal disorders, including inflammatory bowel disease (IBD). This international survey aimed to assess physicians' experiences, perceptions, and practices related to microbiome modulation for gastrointestinal conditions, with a focus on IBD. Results from 142 healthcare professionals, predominantly gastroenterologists, confirmed a consensus on the relevance of the gut microbiota in IBD pathogenesis. However, the utilization of microbial composition analysis and probiotics in clinical practice was limited, primarily due to the lack of standardized guidelines and supporting evidence. Physicians held conflicting views on antibiotics, recognizing their potential for inducing remission but also causing flares in IBD. Respondents also had varying opinions on the efficacy of fecal microbiota transplantation (FMT) for different gastrointestinal conditions, with higher confidence in FMT effectiveness for irritable bowel syndrome with diarrhea, pouchitis, and ulcerative colitis. Concerns on FMT included uncertainty about effect duration, administration intervals, and conflicting evidence. Donor selection was believed to be a crucial factor in FMT outcomes. This survey highlights the need for further research and evidence-based guidelines to optimize the use of microbiome-based therapies in clinical practice. As our understanding of the gut microbiome continues to evolve, these insights will contribute to more informed and personalized approaches to managing gastrointestinal disorders.
Keywords: Crohn’s disease; antibiotics; fecal microbial transplant; inflammatory bowel disease; irritable bowel syndrome; microbiota; pouchitis; probiotics; ulcerative colitis.
Conflict of interest statement
T.L. Parigi has received speaker fees from Abbvie, Ferring, Fresenius Kabi, Takeda, and Tillots. S. Vieujean has received speaker fees from Abbvie, Janssen, Takeda, and Ferring. L. Peyrin-Biroulet reports personal fees from Galapagos, AbbVie, Janssen, Genentech, Ferring, Tillots, Celltrion, Takeda, Pfizer, Index Pharmaceuticals, Sandoz, Celgene, Biogen, Samsung Bioepis, Inotrem, Al-lergan, MSD, Roche, Arena, Gilead, Amgen, BMS, Vifor, Norgine, Mylan, Lilly, Fresenius Kabi, OSE Immunotherapeutics, Enthera, Theravance, Pandion Therapeutics, Gossamer Bio, Viatris, and Thermo Fisher; grants from Abbvie, MSD, Takeda, and Fresenius Kabi; and stock options from CTMA. S. Danese has served as a speaker, consultant, and advisory board member for Schering-Plough, AbbVie, Actelion, Alphawasserman, AstraZeneca, Cellerix, Cosmo Pharmaceuticals, Ferring, Genentech, Grunenthal, Johnson and Johnson, Millenium, Takeda, MSD, Nikkiso Europe GmbH, Novo Nordisk, Nycomed, Pfizer, Pharmacosmos, UCB Pharma, and Vifor. K. Paridaens and K. Dalgaard are employees of Ferring Pharmaceuticals.
Figures
Similar articles
-
Fecal transplantation for treatment of inflammatory bowel disease.Cochrane Database Syst Rev. 2018 Nov 13;11(11):CD012774. doi: 10.1002/14651858.CD012774.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2023 Apr 25;4:CD012774. doi: 10.1002/14651858.CD012774.pub3. PMID: 30480772 Free PMC article. Updated.
-
Optimizing Therapeutic Potential of Fecal Transplant in Inflammatory Bowel Disease.Gastroenterol Clin North Am. 2025 Jun;54(2):277-293. doi: 10.1016/j.gtc.2024.12.002. Epub 2025 Jan 24. Gastroenterol Clin North Am. 2025. PMID: 40348488 Review.
-
Can control of gut microbiota be a future therapeutic option for inflammatory bowel disease?World J Gastroenterol. 2021 Jun 21;27(23):3317-3326. doi: 10.3748/wjg.v27.i23.3317. World J Gastroenterol. 2021. PMID: 34163114 Free PMC article. Review.
-
An update on fecal microbiota transplantation for the treatment of gastrointestinal diseases.J Gastroenterol Hepatol. 2022 Feb;37(2):246-255. doi: 10.1111/jgh.15731. Epub 2021 Nov 12. J Gastroenterol Hepatol. 2022. PMID: 34735024 Review.
-
Gut Microbiota Modulation in IBD: From the Old Paradigm to Revolutionary Tools.Int J Mol Sci. 2025 Mar 27;26(7):3059. doi: 10.3390/ijms26073059. Int J Mol Sci. 2025. PMID: 40243712 Free PMC article. Review.
Cited by
-
Gut Microbiota Metabolites: Unveiling Their Role in Inflammatory Bowel Diseases and Fibrosis.Pharmaceuticals (Basel). 2024 Mar 7;17(3):347. doi: 10.3390/ph17030347. Pharmaceuticals (Basel). 2024. PMID: 38543132 Free PMC article. Review.
-
Mechanisms of microbiota modulation: Implications for health, disease, and therapeutic interventions.Medicine (Baltimore). 2024 May 10;103(19):e38088. doi: 10.1097/MD.0000000000038088. Medicine (Baltimore). 2024. PMID: 38728472 Free PMC article. Review.
-
The Potential Harmful Effects of Genetically Engineered Microorganisms (GEMs) on the Intestinal Microbiome and Public Health.Microorganisms. 2024 Jan 23;12(2):238. doi: 10.3390/microorganisms12020238. Microorganisms. 2024. PMID: 38399642 Free PMC article. Review.
-
Gut microbiota in colorectal cancer: a review of its influence on tumor immune surveillance and therapeutic response.Front Oncol. 2025 Mar 5;15:1557959. doi: 10.3389/fonc.2025.1557959. eCollection 2025. Front Oncol. 2025. PMID: 40110192 Free PMC article. Review.
-
Advancements in Immunomodulatory Therapies for IBD and Their Interplay With the Gut-Brain Axis: An Updated Review of Current Literature and Beyond.Health Sci Rep. 2025 Aug 10;8(8):e71157. doi: 10.1002/hsr2.71157. eCollection 2025 Aug. Health Sci Rep. 2025. PMID: 40791287 Free PMC article.
References
-
- Galeano Niño J.L., Wu H., LaCourse K.D., Kempchinsky A.G., Baryiames A., Barber B., Futran N., Houlton J., Sather C., Sicinska E., et al. Effect of the Intratumoral Microbiota on Spatial and Cellular Heterogeneity in Cancer. Nature. 2022;611:810–817. doi: 10.1038/s41586-022-05435-0. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

