T Cell Response in Tuberculosis-Infected Patients Vaccinated against COVID-19
- PMID: 38004820
- PMCID: PMC10673403
- DOI: 10.3390/microorganisms11112810
T Cell Response in Tuberculosis-Infected Patients Vaccinated against COVID-19
Abstract
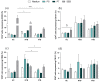
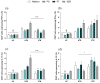
Many studies have focused on SARS-CoV-2 and Mycobacterium tuberculosis (Mtb) co-infection consequences. However, after a vaccination plan against COVID-19, the cases of severe disease and death are consistently controlled, although cases of asymptomatic and mild COVID-19 still happen together with tuberculosis (TB) cases. Thus, in this context, we sought to compare the T cell response of COVID-19-non-vaccinated and -vaccinated patients with active tuberculosis exposed to SARS-CoV-2 antigens. Flow cytometry was used to analyze activation markers (i.e., CD69 and CD137) and cytokines (IFN-γ, TNFα, IL-17, and IL-10) levels in CD4+ and CD8+ T cells upon exposure to SARS-CoV-2 peptides. The data obtained showed that CD8+ T cells from non-vaccinated TB patients present a high frequency of CD69 and TNF-α after viral challenge compared to vaccinated TB donors. Conversely, CD4+ T cells from vaccinated TB patients show a high frequency of IL-10 after spike peptide stimulus compared to non-vaccinated patients. No differences were observed in the other parameters analyzed. The results suggest that this reduced immune balance in coinfected individuals may have consequences for pathogen control, necessitating further research to understand its impact on clinical outcomes after COVID-19 vaccination in those with concurrent SARS-CoV-2 and Mtb infections.
Keywords: immune response; infection; lymphocytes; vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

