Notch Signaling Regulates the Function and Phenotype of Dendritic Cells in Helicobacter pylori Infection
- PMID: 38004829
- PMCID: PMC10673485
- DOI: 10.3390/microorganisms11112818
Notch Signaling Regulates the Function and Phenotype of Dendritic Cells in Helicobacter pylori Infection
Abstract
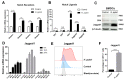
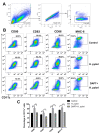
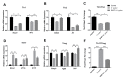
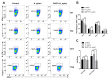
Notch signaling manipulates the function and phenotype of dendritic cells (DCs), as well as the interaction between DCs and CD4+ T cells. However, the role of Notch signaling in Helicobacter pylori (H. pylori) infection remains elusive. Murine bone marrow-derived dendritic cells (BMDCs) were pretreated in the absence or presence of Notch signaling inhibitor DAPT prior to H. pylori stimulation and the levels of Notch components, cytokines and surface markers as well as the differentiation of CD4+ T cells in co-culture were measured using quantitative real-time PCR (qRT-PCR), Western blot, enzyme-linked immunosorbent assay (ELISA) and flow cytometry. Compared with the control, the mRNA expression of all Notch receptors and Notch ligands Dll4 and Jagged1 was up-regulated in H. pylori-stimulated BMDCs. The blockade of Notch signaling by DAPT influenced the production of IL-1β and IL-10 in H. pylori-pulsed BMDCs, and reduced the expression of Notch1, Notch3, Notch4, Dll1, Dll3 and Jagged2. In addition, DAPT pretreatment decreased the expression of maturation markers CD80, CD83, CD86, and major histocompatibility complex class II (MHC-II) of BMDCs, and further skewed Th17/Treg balance toward Treg. Notch signaling regulates the function and phenotype of DCs, thus mediating the differentiation of CD4+ T cells during H. pylori infection.
Keywords: CD4+ T cells; Helicobacter pylori; Notch signaling; dendritic cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hooi J.K.Y., Lai W.Y., Ng W.K., Suen M.M.Y., Underwood F.E., Tanyingoh D., Malfertheiner P., Graham D.Y., Wong V.W.S., Wu J.C.Y., et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. - DOI - PubMed
-
- Kao J.Y., Zhang M., Miller M.J., Mills J.C., Wang B., Liu M., Eaton K.A., Zou W., Berndt B.E., Cole T.S., et al. Helicobacter pylori Immune Escape Is Mediated by Dendritic Cell–Induced Treg Skewing and Th17 Suppression in Mice. Gastroenterology. 2010;138:1046–1054. doi: 10.1053/j.gastro.2009.11.043. - DOI - PMC - PubMed
Grants and funding
- No. 81971903/National Natural Science Foundation of China
- No. 2019YFA0903802/National Key Research and Development Project of China
- No.2023A1515010084/Basic and Applied basic Research Project of Guangdong Province
- S202212121162,202212121322/Innovative Experiment Program of College Students of Guangdong Province, China
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

