Peculiarity of the Structure and Luminescence of Glasses in La2S3-Ga2S3-GeS2:Pr3+ System
- PMID: 38005024
- PMCID: PMC10672385
- DOI: 10.3390/ma16227094
Peculiarity of the Structure and Luminescence of Glasses in La2S3-Ga2S3-GeS2:Pr3+ System
Abstract
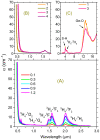
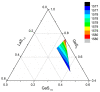
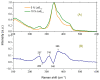
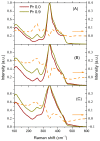
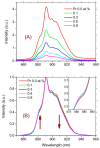
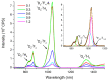
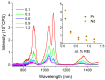
The effect of modifying the composition of a glass matrix based on the Ga2S3-GeS2:Pr3+ system due to the addition of La2S3 on the structure and the optical and luminescent properties of these glasses has been studied. It has been shown that the addition of La2S3 leads to changes in the nearest structural environment of Ga, Ge, and S and increases the degree of ionicity of the bonds of the Pr3+ ion. Despite the existence of a large glass formation region in the Ga2S3-GeS2-La2S3 system and the structural and chemical similarity of La and Pr, La2S3 does not promote a more uniform distribution of Pr3+ ions in the glass matrix, and thus does not reduce the concentration quenching of the luminescence of Pr3+ ions. However, the addition of La2S3 increases the probability of emission of Pr3+ ions and decreases the radiative lifetime. Additionally, it was shown that, when studying the structure and luminescent properties of glasses with La, it is necessary to take into account a significant concentration of rare earth traces (Pr and Nd).
Keywords: IR materials; chalcogenide glasses; luminescent materials.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Guo H., Cui J., Xu C., Xu Y., Farrell G. Mid-Infrared Fluoride and Chalcogenide Glasses and Fibers. Progress in Optical Science and Photonics. Volume 18. Springer; Singapore: 2022. Mid-Infrared Spectral Properties of Rare Earth Ion Doped Chalcogenide Glasses and Fibers. - DOI
-
- Bensaid S., Kachenoura A., Costet N., Bensalah K., Tariel H., Senhadji L. Noninvasive detection of bladder cancer using mid-infrared spectra classification. Expert Syst. Appl. 2017;89:333–342. doi: 10.1016/j.eswa.2017.07.052. - DOI
-
- Lucas P., Coleman G.J., Jiang S.B., Luo T., Yang Z.Y. Chalcogenide glass fibers: Optical window tailoring and suitability for bio-chemical sensing. Opt. Mater. 2015;47:530–536. doi: 10.1016/j.optmat.2015.06.034. - DOI
-
- Kumta P.N., Risbud S.H. Rare-earth chalcogenides—An emerging class of optical materials. J. Mater. Sci. 1994;29:1135–1158. doi: 10.1007/BF00975057. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

