Advancing Our Understanding of Pyranopterin-Dithiolene Contributions to Moco Enzyme Catalysis
- PMID: 38005178
- PMCID: PMC10673323
- DOI: 10.3390/molecules28227456
Advancing Our Understanding of Pyranopterin-Dithiolene Contributions to Moco Enzyme Catalysis
Abstract
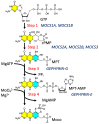
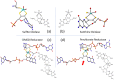
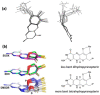
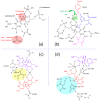
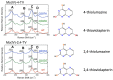
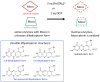
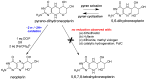
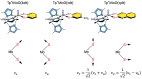
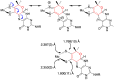
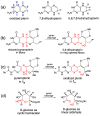
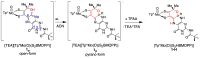
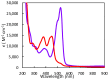
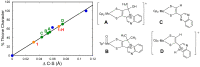
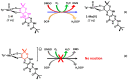
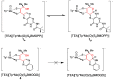
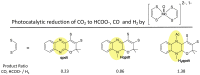
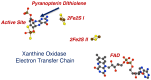
The pyranopterin dithiolene ligand is remarkable in terms of its geometric and electronic structure and is uniquely found in mononuclear molybdenum and tungsten enzymes. The pyranopterin dithiolene is found coordinated to the metal ion, deeply buried within the protein, and non-covalently attached to the protein via an extensive hydrogen bonding network that is enzyme-specific. However, the function of pyranopterin dithiolene in enzymatic catalysis has been difficult to determine. This focused account aims to provide an overview of what has been learned from the study of pyranopterin dithiolene model complexes of molybdenum and how these results relate to the enzyme systems. This work begins with a summary of what is known about the pyranopterin dithiolene ligand in the enzymes. We then introduce the development of inorganic small molecule complexes that model aspects of a coordinated pyranopterin dithiolene and discuss the results of detailed physical studies of the models by electronic absorption, resonance Raman, X-ray absorption and NMR spectroscopies, cyclic voltammetry, X-ray crystallography, and chemical reactivity.
Keywords: Moco; dithiolene; molybdenum cofactor; molybdenum enzymes; molybdopterin; pyranopterin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
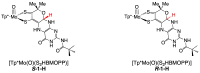
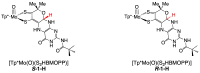
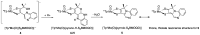
References
-
- Ingersol L.J., Kirk M.L. Structure, Function, and Mechanism of Pyranopterin Molybdenum and Tungsten Enzymes. In: Constable E.C., Parkin G., Que L. Jr., editors. Comprehensive Coordination Chemistry III. Elsevier; Oxford, UK: 2021. pp. 790–811.
-
- Hille R., Schulzke C., Kirk M.L. Molybdenum and Tungsten Enzymes. The Royal Society of Chemistry; Cambridge, UK: 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

