Anti- Trypanosoma cruzi Activity, Mutagenicity, Hepatocytotoxicity and Nitroreductase Enzyme Evaluation of 3-Nitrotriazole, 2-Nitroimidazole and Triazole Derivatives
- PMID: 38005183
- PMCID: PMC10672842
- DOI: 10.3390/molecules28227461
Anti- Trypanosoma cruzi Activity, Mutagenicity, Hepatocytotoxicity and Nitroreductase Enzyme Evaluation of 3-Nitrotriazole, 2-Nitroimidazole and Triazole Derivatives
Abstract
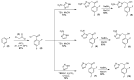
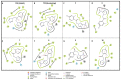
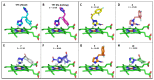
Chagas disease (CD), which is caused by Trypanosoma cruzi and was discovered more than 100 years ago, remains the leading cause of death from parasitic diseases in the Americas. As a curative treatment is only available for the acute phase of CD, the search for new therapeutic options is urgent. In this study, nitroazole and azole compounds were synthesized and underwent molecular modeling, anti-T. cruzi evaluations and nitroreductase enzymatic assays. The compounds were designed as possible inhibitors of ergosterol biosynthesis and/or as substrates of nitroreductase enzymes. The in vitro evaluation against T. cruzi clearly showed that nitrotriazole compounds are significantly more potent than nitroimidazoles and triazoles. When their carbonyls were reduced to hydroxyl groups, the compounds showed a significant increase in activity. In addition, these substances showed potential for action via nitroreductase activation, as the substances were metabolized at higher rates than benznidazole (BZN), a reference drug against CD. Among the compounds, 1-(2,4-difluorophenyl)-2-(3-nitro-1H-1,2,4-triazol-1-yl)ethanol (8) is the most potent and selective of the series, with an IC50 of 0.39 µM and selectivity index of 3077; compared to BZN, 8 is 4-fold more potent and 2-fold more selective. Moreover, this compound was not mutagenic at any of the concentrations evaluated, exhibited a favorable in silico ADMET profile and showed a low potential for hepatotoxicity, as evidenced by the high values of CC50 in HepG2 cells. Furthermore, compared to BZN, derivative 8 showed a higher rate of conversion by nitroreductase and was metabolized three times more quickly when both compounds were tested at a concentration of 50 µM. The results obtained by the enzymatic evaluation and molecular docking studies suggest that, as planned, nitroazole derivatives may utilize the nitroreductase metabolism pathway as their main mechanism of action against Trypanosoma cruzi. In summary, we have successfully identified and characterized new nitrotriazole analogs, demonstrating their potential as promising candidates for the development of Chagas disease drug candidates that function via nitroreductase activation, are considerably selective and show no mutagenic potential.
Keywords: CYP51; Chagas disease; Trypanosoma cruzi; azole; heterocycle; imidazole; nitroreductase; triazole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Chagas C. Nova Entidade Morbida Do Homem: Rezumo Geral de Estudos Etiolojicos e Clinicos. Mem. Inst. Oswaldo Cruz. 1911;3:219–275. doi: 10.1590/S0074-02761911000200003. - DOI
-
- WHO Chagas Disease (American Trypanosomiasis) [(accessed on 6 July 2023)]. Available online: https://www.who.int/health-topics/chagas-disease#tab=tab_1.
-
- Cirqueira M.L., Bortot L.O., Bolean M., Aleixo M.A.A., Luccas P.H., Costa-Filho A.J., Ramos A.P., Ciancaglini P., Nonato M.C. Trypanosoma Cruzi Nitroreductase: Structural Features and Interaction with Biological Membranes. Int. J. Biol. Macromol. 2022;221:891–899. doi: 10.1016/j.ijbiomac.2022.09.073. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- E-26/202.707/2018/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- E-26/202.805/2017/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- E-26/200.535/2020/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- E-26/202.759/2017/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- E-26/010.002119/2019/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- E-26/010.100956/2018/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- 140113/2022-3/National Council for Scientific and Technological Development
- 302345/2017-5/National Council for Scientific and Technological Development
- 407852/2017-4/National Council for Scientific and Technological Development
- 440013/2022-4/National Council for Scientific and Technological Development
- 306193/2018-3/National Council for Scientific and Technological Development
- 420351/2016-7/National Council for Scientific and Technological Development
- 312288/2017-4/National Council for Scientific and Technological Development
- 315480/2020-3/National Council for Scientific and Technological Development
- 2016/São Paulo Research Foundation
LinkOut - more resources
Full Text Sources
Medical
Research Materials

