The Phenolic Profile and Anti-Inflammatory Effect of Ethanolic Extract of Polish Propolis on Activated Human Gingival Fibroblasts-1 Cell Line
- PMID: 38005199
- PMCID: PMC10673102
- DOI: 10.3390/molecules28227477
The Phenolic Profile and Anti-Inflammatory Effect of Ethanolic Extract of Polish Propolis on Activated Human Gingival Fibroblasts-1 Cell Line
Abstract
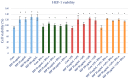
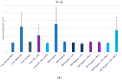
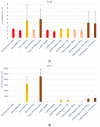
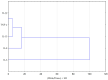
Propolis, owing to its antibacterial and anti-inflammatory properties, acts as a cariostatic agent, capable of preventing the accumulation of dental plaque and inhibiting inflammation. The anti-inflammatory properties of propolis are attributed to caffeic acid phenethyl ester (CAPE), which is present in European propolis. The objective of the conducted study was to assess the anti-inflammatory effects of the Polish ethanolic extract of propolis (EEP) and isolated CAPE on stimulated with LPS and IFN-α, as well as the combination of LPS and IFN-α. The cytotoxicity of the tested compounds was determined using the MTT assay. The concentrations of specific cytokines released by the HGF-1 cell line following treatment with EEP (25-50 µg/mL) or CAPE (25-50 µg/mL) were assessed in the culture supernatant. In the tested concentrations, both CAPE and EEP did not exert cytotoxic effects. Our results demonstrate that CAPE reduces TNF-α and IL-6 in contrast to EEP. Propolis seems effective in stimulating HGF-1 to release IL-6 and IL-8. A statistically significant difference was observed for IL-8 in HGF-1 stimulated by LPS+IFN-α and treated EEP at a concentration of 50 µg/mL (p = 0.021201). Moreover, we observed that CAPE demonstrates a stronger interaction with IL-8 compared to EEP, especially when CAPE was administered at a concentration of 50 µg/mL after LPS + IFN-α stimulation (p = 0.0005). Analysis of the phenolic profile performed by high-performance liquid chromatography allowed identification and quantification in the EEP sample of six phenolic acids, five flavonoids, and one aromatic ester-CAPE. Propolis and its compound-CAPE-exhibit immunomodulatory properties that influence the inflammatory process. Further studies may contribute to explaining the immunomodulatory action of EEP and CAPE and bring comprehensive conclusions.
Keywords: caffeic acid phenethyl ester; cytokines; in vitro model; inflammation; polyphenols; propolis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Kurek-Górecka A., Walczyńska-Dragon K., Felitti R., Nitecka-Buchta A., Baron S., Olczyk P. The Influence of Propolis on Dental Plaque Reduction and the Correlation between Dental Plaque and Severity of COVID-19 Complications—A Literature Review. Molecules. 2021;26:5516. doi: 10.3390/molecules26185516. - DOI - PMC - PubMed
-
- Gündogar H., Üstün K., Şenyurt S.Z., Özdemir E.Ç., Sezer U., Erciyas K. Gingival crevicular fluid levels of cytokine, chemokine, and growth factors in patients with periodontitis or gingivitis and periodontally healthy subjects: A cross-sectional multiplex study. Central Eur. J. Immunol. 2021;46:474–480. doi: 10.5114/ceji.2021.110289. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

