Density Functional Theory and Density Functional Tight Binding Studies of Thiamine Hydrochloride Hydrates
- PMID: 38005219
- PMCID: PMC10673443
- DOI: 10.3390/molecules28227497
Density Functional Theory and Density Functional Tight Binding Studies of Thiamine Hydrochloride Hydrates
Abstract
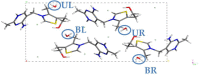
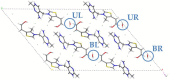
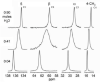
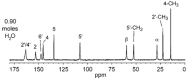
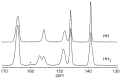
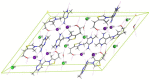
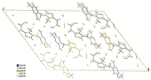
Thiamine hydrochloride (THCL), also known as vitamin B1, is an active pharmaceutical ingredient (API), present on the list of essential medicines developed by the WHO, which proves its importance for public health. THCL is highly hygroscopic and can occur in the form of hydrates with varying degrees of hydration, depending on the air humidity. Although experimental characterization of the THCL hydrates has been described in the literature, the questions raised in previously published works suggest that additional research and in-depth analysis of THCL dehydration behavior are still needed. Therefore, the main aim of this study was to characterize, by means of quantum chemical calculations, the behavior of thiamine hydrates and explain the previously obtained results, including changes in the NMR spectra, at the molecular level. To achieve this goal, a series of DFT (CASTEP) and DFTB (DFTB+) calculations under periodic boundary conditions have been performed, including molecular dynamics simulations and GIPAW NMR calculations. The obtained results explain the differences in the relative stability of the studied forms and changes in the spectra observed for the samples of various degrees of hydration. This work highlights the application of periodic DFT calculations in the analysis of various solid forms of APIs.
Keywords: CASTEP; DFT; DFTB; GIPAW; hydrate; ssNMR; thiamine hydrochloride.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of this study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
Hydrates of active pharmaceutical ingredients: A 35Cl and 2H solid-state NMR and DFT study.Solid State Nucl Magn Reson. 2022 Dec;122:101837. doi: 10.1016/j.ssnmr.2022.101837. Epub 2022 Nov 4. Solid State Nucl Magn Reson. 2022. PMID: 36434925
-
Insights into the dehydration behavior of thiamine hydrochloride (vitamin B1) hydrates: part I.J Pharm Sci. 2010 Feb;99(2):816-27. doi: 10.1002/jps.21876. J Pharm Sci. 2010. PMID: 19623540
-
Insights into the dehydration behavior of thiamine hydrochloride (vitamin B1) hydrates: part II.J Pharm Sci. 2010 Apr;99(4):1882-95. doi: 10.1002/jps.21968. J Pharm Sci. 2010. PMID: 19824063
-
Periodic DFT Calculations-Review of Applications in the Pharmaceutical Sciences.Pharmaceutics. 2020 May 1;12(5):415. doi: 10.3390/pharmaceutics12050415. Pharmaceutics. 2020. PMID: 32369915 Free PMC article. Review.
-
The PAW/GIPAW approach for computing NMR parameters: a new dimension added to NMR study of solids.Solid State Nucl Magn Reson. 2011 Jul;40(1):1-20. doi: 10.1016/j.ssnmr.2011.04.006. Epub 2011 May 7. Solid State Nucl Magn Reson. 2011. PMID: 21612895 Review.
Cited by
-
Solvent Effect on Cation⊗3π Interactions: A First-Principles Study.Molecules. 2024 Oct 29;29(21):5099. doi: 10.3390/molecules29215099. Molecules. 2024. PMID: 39519740 Free PMC article.
References
-
- Brog J.-P., Chanez C.-L., Crochet A., Fromm K.M. Polymorphism, What It Is and How to Identify It: A Systematic Review. RSC Adv. 2013;3:16905. doi: 10.1039/c3ra41559g. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

