Two-Dimensional-PAGE Coupled with nLC-MS/MS-Based Identification of Differentially Expressed Proteins and Tumorigenic Pathways in MCF7 Breast Cancer Cells Transfected for JTB Protein Silencing
- PMID: 38005222
- PMCID: PMC10673289
- DOI: 10.3390/molecules28227501
Two-Dimensional-PAGE Coupled with nLC-MS/MS-Based Identification of Differentially Expressed Proteins and Tumorigenic Pathways in MCF7 Breast Cancer Cells Transfected for JTB Protein Silencing
Abstract
The identification of new cancer-associated genes/proteins, the characterization of their expression variation, the interactomics-based assessment of differentially expressed genes/proteins (DEGs/DEPs), and understanding the tumorigenic pathways and biological processes involved in BC genesis and progression are necessary and possible by the rapid and recent advances in bioinformatics and molecular profiling strategies. Taking into account the opinion of other authors, as well as based on our own team's in vitro studies, we suggest that the human jumping translocation breakpoint (hJTB) protein might be considered as a tumor biomarker for BC and should be studied as a target for BC therapy. In this study, we identify DEPs, carcinogenic pathways, and biological processes associated with JTB silencing, using 2D-PAGE coupled with nano-liquid chromatography tandem mass spectrometry (nLC-MS/MS) proteomics applied to a MCF7 breast cancer cell line, for complementing and completing our previous results based on SDS-PAGE, as well as in-solution proteomics of MCF7 cells transfected for JTB downregulation. The functions of significant DEPs are analyzed using GSEA and KEGG analyses. Almost all DEPs exert pro-tumorigenic effects in the JTBlow condition, sustaining the tumor suppressive function of JTB. Thus, the identified DEPs are involved in several signaling and metabolic pathways that play pro-tumorigenic roles: EMT, ERK/MAPK, PI3K/AKT, Wnt/β-catenin, mTOR, C-MYC, NF-κB, IFN-γ and IFN-α responses, UPR, and glycolysis/gluconeogenesis. These pathways sustain cancer cell growth, adhesion, survival, proliferation, invasion, metastasis, resistance to apoptosis, tight junctions and cytoskeleton reorganization, the maintenance of stemness, metabolic reprogramming, survival in a hostile environment, and sustain a poor clinical outcome. In conclusion, JTB silencing might increase the neoplastic phenotype and behavior of the MCF7 BC cell line. The data is available via ProteomeXchange with the identifier PXD046265.
Keywords: JTB protein silencing; MCF7; breast cancer (BC); downregulated JTB interactome; overexpressed JTB interactome; tumorigenic pathways.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

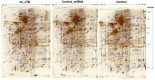

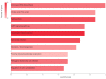

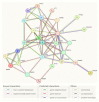

Similar articles
-
Two-Dimensional Polyacrylamide Gel Electrophoresis Coupled with Nanoliquid Chromatography-Tandem Mass Spectrometry-Based Identification of Differentially Expressed Proteins and Tumorigenic Pathways in the MCF7 Breast Cancer Cell Line Transfected for Jumping Translocation Breakpoint Protein Overexpression.Int J Mol Sci. 2023 Sep 28;24(19):14714. doi: 10.3390/ijms241914714. Int J Mol Sci. 2023. PMID: 37834160 Free PMC article.
-
Investigating the Function of Human Jumping Translocation Breakpoint Protein (hJTB) and Its Interacting Partners through In-Solution Proteomics of MCF7 Cells.Molecules. 2022 Nov 28;27(23):8301. doi: 10.3390/molecules27238301. Molecules. 2022. PMID: 36500393 Free PMC article.
-
Investigation of the effects of overexpression of jumping translocation breakpoint (JTB) protein in MCF7 cells for potential use as a biomarker in breast cancer.Am J Cancer Res. 2022 Apr 15;12(4):1784-1823. eCollection 2022. Am J Cancer Res. 2022. PMID: 35530281 Free PMC article.
-
Molecular mechanisms underlying the action of carcinogens in gastric cancer with a glimpse into targeted therapy.Cell Oncol (Dordr). 2022 Dec;45(6):1073-1117. doi: 10.1007/s13402-022-00715-3. Epub 2022 Sep 23. Cell Oncol (Dordr). 2022. PMID: 36149600 Review.
-
Brain metastasis in breast cancer: focus on genes and signaling pathways involved, blood-brain barrier and treatment strategies.Clin Transl Oncol. 2023 May;25(5):1218-1241. doi: 10.1007/s12094-022-03050-z. Epub 2023 Mar 10. Clin Transl Oncol. 2023. PMID: 36897508 Review.
Cited by
-
From Jumping Gene to Cancer: Revisiting the Role of JTB Protein.Biomedicines. 2025 Jul 12;13(7):1705. doi: 10.3390/biomedicines13071705. Biomedicines. 2025. PMID: 40722776 Free PMC article. Review.
References
-
- Tyszkiewicz T., Jarzab M., Szymczyk C., Kowal M., Krajewska J., Jaworska M., Fraczek M., Krajewska A., Hadas E., Swierniak M., et al. Epidermal differentiation complex (locus 1q21) gene expression in head and neck cancer and normal mucosa. Folia Histochem. et Cytobiol. 2014;52:79–89. doi: 10.5603/FHC.2014.0018. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

