Sulconazole-Loaded Solid Lipid Nanoparticles for Enhanced Antifungal Activity: In Vitro and In Vivo Approach
- PMID: 38005230
- PMCID: PMC10672792
- DOI: 10.3390/molecules28227508
Sulconazole-Loaded Solid Lipid Nanoparticles for Enhanced Antifungal Activity: In Vitro and In Vivo Approach
Abstract
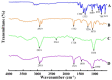
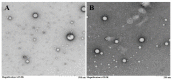
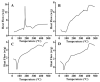
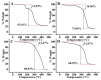
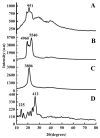
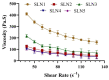
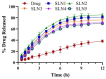
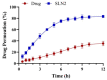
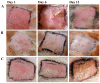
Solid lipid nanoparticles (SLNs) have the advantages of a cell-specific delivery and sustained release of hydrophobic drugs that can be exploited against infectious diseases. The topical delivery of hydrophobic drugs needs pharmaceutical strategies to enhance drug permeation, which is a challenge faced by conventional formulations containing a drug suspended in gel, creams or ointments. We report the fabrication and optimization of SLNs with sulconazole (SCZ) as a model hydrophobic drug and then a formulation of an SLN-based topical gel against fungal infections. The SLNs were optimized through excipients of glyceryl monostearate and Phospholipon® 90 H as lipids and tween 20 as a surfactant for its size, drug entrapment and sustained release and resistance against aggregation. The SCZ-SLNs were physically characterized for their particle size (89.81 ± 2.64), polydispersity index (0.311 ± 0.07), zeta potential (-26.98 ± 1.19) and encapsulation efficiency (86.52 ± 0.53). The SCZ-SLNs showed sustained release of 85.29% drug at the 12 h timepoint. The TEM results demonstrated spherical morphology, while DSC, XRD and FTIR showed the compatibility of the drug inside SLNs. SCZ-SLNs were incorporated into a gel using carbopol and were further optimized for their rheological behavior, pH, homogeneity and spreadability on the skin. The antifungal activity against Candida albicans and Trichophyton rubrum was increased in comparison to a SCZ carbopol-based gel. In vivo antifungal activity in rabbits presented faster healing of skin fungal infections. The histopathological examination of the treated skin from rabbits presented restoration of the dermal architecture. In summary, the approach of formulating SLNs into a topical gel presented an advantageous drug delivery system against mycosis.
Keywords: anti-fungal gel; histopathology; solid lipid nanoparticles; sulconazole.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

Similar articles
-
Sucrose ester stabilized solid lipid nanoparticles and nanostructured lipid carriers. II. Evaluation of the imidazole antifungal drug-loaded nanoparticle dispersions and their gel formulations.Nanotechnology. 2014 Mar 14;25(10):105102. doi: 10.1088/0957-4484/25/10/105102. Epub 2014 Feb 14. Nanotechnology. 2014. PMID: 24531828
-
Fluconazole-loaded solid lipid nanoparticles topical gel for treatment of pityriasis versicolor: formulation and clinical study.Drug Deliv. 2018 Nov;25(1):78-90. doi: 10.1080/10717544.2017.1413444. Drug Deliv. 2018. PMID: 29239242 Free PMC article. Clinical Trial.
-
Development of solid lipid nanoparticles based controlled release system for topical delivery of terbinafine hydrochloride.Eur J Pharm Sci. 2013 May 13;49(2):311-22. doi: 10.1016/j.ejps.2013.03.013. Epub 2013 Apr 2. Eur J Pharm Sci. 2013. PMID: 23557842
-
Solid lipid nanoparticles and their application in the treatment of bacterial infectious diseases.Biomed Pharmacother. 2024 May;174:116433. doi: 10.1016/j.biopha.2024.116433. Epub 2024 Mar 19. Biomed Pharmacother. 2024. PMID: 38508079 Review.
-
Recent Progression in Nanocarrier based Techniques to Address Fungal Infections and Patent Status in Drug Development Process.Recent Pat Nanotechnol. 2025;19(2):183-204. doi: 10.2174/1872210517666230727090314. Recent Pat Nanotechnol. 2025. PMID: 37497703 Review.
Cited by
-
Optimization of atorvastatin and quercetin-loaded solid lipid nanoparticles using Box-Behnken design.Nanomedicine (Lond). 2024 Jul 14;19(17):1541-1555. doi: 10.1080/17435889.2024.2364585. Epub 2024 Jul 16. Nanomedicine (Lond). 2024. PMID: 39012199 Free PMC article.
-
Cannabidiol-Loaded Solid Lipid Nanoparticles Ameliorate the Inhibition of Proinflammatory Cytokines and Free Radicals in an In Vitro Inflammation-Induced Cell Model.Int J Mol Sci. 2024 Apr 26;25(9):4744. doi: 10.3390/ijms25094744. Int J Mol Sci. 2024. PMID: 38731964 Free PMC article.
-
Progress in Topical and Transdermal Drug Delivery Research-Focus on Nanoformulations.Pharmaceutics. 2024 Jun 16;16(6):817. doi: 10.3390/pharmaceutics16060817. Pharmaceutics. 2024. PMID: 38931938 Free PMC article. Review.
References
-
- Tanitame A., Oyamada Y., Ofuji K., Fujimoto M., Suzuki K., Ueda T., Terauchi H., Kawasaki M., Nagai K., Wachi M., et al. Synthesis and antibacterial activity of novel and potent DNA gyrase inhibitors with azole ring. Bioorg. Med. Chem. 2004;12:5515–5524. doi: 10.1016/j.bmc.2004.08.010. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

