Research Advances in Clinical Applications, Anticancer Mechanism, Total Chemical Synthesis, Semi-Synthesis and Biosynthesis of Paclitaxel
- PMID: 38005238
- PMCID: PMC10673093
- DOI: 10.3390/molecules28227517
Research Advances in Clinical Applications, Anticancer Mechanism, Total Chemical Synthesis, Semi-Synthesis and Biosynthesis of Paclitaxel
Abstract
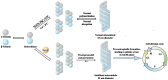
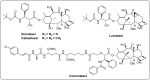
Paclitaxel, a natural secondary metabolite isolated and purified from the bark of the Taxus tree, is considered one of the most successful natural anticancer drugs due to its low toxicity, high potency and broad-spectrum anticancer activity. Taxus trees are scarce and slow-growing, and with extremely low paclitaxel content, the contradiction between supply and demand in the market is becoming more and more intense. Therefore, researchers have tried to obtain paclitaxel by various methods such as chemical synthesis, artificial culture, microbial fermentation and tissue cell culture to meet the clinical demand for this drug. This paper provides a comprehensive overview of paclitaxel extraction, combination therapy, total synthesis, semi-synthesis and biosynthesis in recent years and provides an outlook, aiming to provide a theoretical basis and reference for further research on the production and application of paclitaxel in the future.
Keywords: anticancer mechanism; biosynthesis; paclitaxel; semi-synthesis; total synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
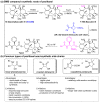

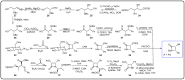



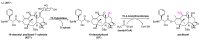
References
-
- Perez-Matas E., Hanano A., Moyano E., Bonfill M., Cusido R.M., Palazon J. Insights into the control of taxane metabolism: Molecular, cellular, and metabolic changes induced by elicitation in Taxus baccata cell suspensions. Front. Plant Sci. 2022;13:942433. doi: 10.3389/fpls.2022.942433. - DOI - PMC - PubMed
-
- Zefirova O.N., Nurieva E.V., Ryzhov A.N., Zyk N.V., Zefirov N.S. Taxol: Synthesis, Bioactive Conformations, and Structure-Activity Relationships in Its Analogs. Russ. J. Org. Chem. 2005;41:315–351. doi: 10.1007/s11178-005-0168-0. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

