Study Models of Drug-Drug Interactions Involving P-Glycoprotein: The Potential Benefit of P-Glycoprotein Modulation at the Kidney and Intestinal Levels
- PMID: 38005253
- PMCID: PMC10673607
- DOI: 10.3390/molecules28227532
Study Models of Drug-Drug Interactions Involving P-Glycoprotein: The Potential Benefit of P-Glycoprotein Modulation at the Kidney and Intestinal Levels
Abstract
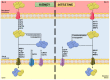
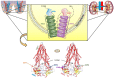
P-glycoprotein (P-gp) is a crucial membrane transporter situated on the cell's apical surface, being responsible for eliminating xenobiotics and endobiotics. P-gp modulators are compounds that can directly or indirectly affect this protein, leading to changes in its expression and function. These modulators can act as inhibitors, inducers, or activators, potentially causing drug-drug interactions (DDIs). This comprehensive review explores diverse models and techniques used to assess drug-induced P-gp modulation. We cover several approaches, including in silico, in vitro, ex vivo, and in vivo methods, with their respective strengths and limitations. Additionally, we explore the therapeutic implications of DDIs involving P-gp, with a special focus on the renal and intestinal elimination of P-gp substrates. This involves enhancing the removal of toxic substances from proximal tubular epithelial cells into the urine or increasing the transport of compounds from enterocytes into the intestinal lumen, thereby facilitating their excretion in the feces. A better understanding of these interactions, and of the distinct techniques applied for their study, will be of utmost importance for optimizing drug therapy, consequently minimizing drug-induced adverse and toxic effects.
Keywords: P-glycoprotein; activation; ex vivo; in silico; in vitro; in vivo; induction; inhibition; kidney.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

