Exploring Hydrogen Sources in Catalytic Transfer Hydrogenation: A Review of Unsaturated Compound Reduction
- PMID: 38005261
- PMCID: PMC10673347
- DOI: 10.3390/molecules28227541
Exploring Hydrogen Sources in Catalytic Transfer Hydrogenation: A Review of Unsaturated Compound Reduction
Abstract
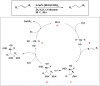
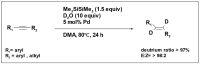
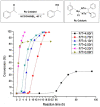
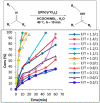
Catalytic transfer hydrogenation has emerged as a pivotal chemical process with transformative potential in various industries. This review highlights the significance of catalytic transfer hydrogenation, a reaction that facilitates the transfer of hydrogen from one molecule to another, using a distinct molecule as the hydrogen source in the presence of a catalyst. Unlike conventional direct hydrogenation, catalytic transfer hydrogenation offers numerous advantages, such as enhanced safety, cost-effective hydrogen donors, byproduct recyclability, catalyst accessibility, and the potential for catalytic asymmetric transfer hydrogenation, particularly with chiral ligands. Moreover, the diverse range of hydrogen donor molecules utilized in this reaction have been explored, shedding light on their unique properties and their impact on catalytic systems and the mechanism elucidation of some reactions. Alcohols such as methanol and isopropanol are prominent hydrogen donors, demonstrating remarkable efficacy in various reductions. Formic acid offers irreversible hydrogenation, preventing the occurrence of reverse reactions, and is extensively utilized in chiral compound synthesis. Unconventional donors such as 1,4-cyclohexadiene and glycerol have shown a good efficiency in reducing unsaturated compounds, with glycerol additionally serving as a green solvent in some transformations. The compatibility of these donors with various catalysts, substrates, and reaction conditions were all discussed. Furthermore, this paper outlines future trends which include the utilization of biomass-derived hydrogen donors, the exploration of hydrogen storage materials such as metal-organic frameworks (MOFs), catalyst development for enhanced activity and recyclability, and the utilization of eco-friendly solvents such as glycerol and ionic liquids. Innovative heating methods, diverse base materials, and continued research into catalyst-hydrogen donor interactions are aimed to shape the future of catalytic transfer hydrogenation, enhancing its selectivity and efficiency across various industries and applications.
Keywords: 1,4-cyclohexadiene; alcohol; amine; formic acid; glycerol; hydrogen donor molecules; hydrogen transfer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







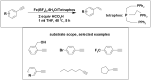

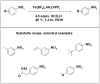






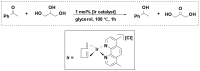
References
-
- Whitner T.C. Review of hydrogenation. Oil Soap. 1939;16:39–44. doi: 10.1007/BF02543211. - DOI
-
- Baráth E. Hydrogen Transfer Reactions of Carbonyls, Alkynes, and Alkenes with Noble Metals in the Presence of Alcohols/Ethers and Amines as Hydrogen Donors. Catalysts. 2018;8:671. doi: 10.3390/catal8120671. - DOI
-
- Meerwein H., Schmidt R. Ein neues Verfahren zur Reduktion von Aldehyden und Ketonen. Justus Liebig Ann. Der Chem. 1925;444:221–238. doi: 10.1002/jlac.19254440112. - DOI
-
- Verley A. The exchange of functional groups between two molecules. The passage of ketones to alcohols and the reverse. Bull. Soc. Chim. Fr. 1925;37:871–874.
-
- Chuah G., Jaenicke S., Zhu Y., Liu S. Meerwein-Ponndorf-Verley Reduction over Heterogeneous Catalysts. Curr. Org. Chem. 2006;10:1639–1654. doi: 10.2174/138527206778249621. - DOI
Publication types
LinkOut - more resources
Full Text Sources

