A Spectroscopic and Molecular Dynamics Study on the Aggregation Properties of a Lipopeptide Analogue of Liraglutide, a Therapeutic Peptide against Diabetes Type 2
- PMID: 38005270
- PMCID: PMC10674484
- DOI: 10.3390/molecules28227536
A Spectroscopic and Molecular Dynamics Study on the Aggregation Properties of a Lipopeptide Analogue of Liraglutide, a Therapeutic Peptide against Diabetes Type 2
Abstract
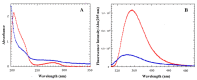
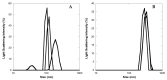
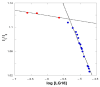
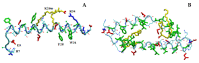
The pharmacokinetics of peptide drugs are strongly affected by their aggregation properties and the morphology of the nanostructures they form in their native state as well as in their therapeutic formulation. In this contribution, we analyze the aggregation properties of a Liraglutide analogue (LG18), a leading drug against diabetes type 2. LG18 is a lipopeptide characterized by the functionalization of a lysine residue (K26) with an 18C lipid chain. To this end, spectroscopic experiments, dynamic light scattering measurements, and molecular dynamics simulations were carried out, following the evolution of the aggregation process from the small LG18 clusters formed at sub-micromolar concentrations to the mesoscopic aggregates formed by aged micromolar solutions. The critical aggregation concentration of LG18 in water (pH = 8) was found to amount to 4.3 μM, as assessed by the pyrene fluorescence assay. MD simulations showed that the LG18 nanostructures are formed by tetramer building blocks that, at longer times, self-assemble to form micrometric supramolecular architectures.
Keywords: liraglutide analogue; molecular dynamics simulations; peptide aggregation; peptide nanostructures; therapeutic peptides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
A spectroscopic and molecular dynamics study on the aggregation process of a long-acting lipidated therapeutic peptide: the case of semaglutide.Soft Matter. 2020 Nov 18;16(44):10122-10131. doi: 10.1039/d0sm01011a. Soft Matter. 2020. PMID: 32780784
-
Semaglutide Aggregates into Oligomeric Micelles and Short Fibrils in Aqueous Solution.Biomacromolecules. 2025 Jun 9;26(6):3786-3794. doi: 10.1021/acs.biomac.5c00342. Epub 2025 May 12. Biomacromolecules. 2025. PMID: 40355389 Free PMC article.
-
Application of Af4-Multidetection to Liraglutide in Its Formulation: Preserving and Representing Native Aggregation.Molecules. 2022 Aug 26;27(17):5485. doi: 10.3390/molecules27175485. Molecules. 2022. PMID: 36080254 Free PMC article.
-
Liraglutide in Type 2 Diabetes Mellitus: Clinical Pharmacokinetics and Pharmacodynamics.Clin Pharmacokinet. 2016 Jun;55(6):657-72. doi: 10.1007/s40262-015-0343-6. Clin Pharmacokinet. 2016. PMID: 26597252 Free PMC article. Review.
-
Liraglutide: a once-daily GLP-1 analogue for the treatment of type 2 diabetes mellitus.Expert Opin Investig Drugs. 2007 Feb;16(2):231-7. doi: 10.1517/13543784.16.2.231. Expert Opin Investig Drugs. 2007. PMID: 17243943 Review.
Cited by
-
Effect of Lipidation on the Structure, Oligomerization, and Aggregation of Glucagon-like Peptide 1.Bioconjug Chem. 2025 Mar 19;36(3):401-414. doi: 10.1021/acs.bioconjchem.4c00484. Epub 2025 Jan 22. Bioconjug Chem. 2025. PMID: 39841169 Free PMC article.
References
-
- Brown T.D., Whitehead K.A., Mitragotri S. Materials for oral delivery of proteins and peptides. Nat. Rev. Mater. 2020;5:127–148. doi: 10.1038/s41578-019-0156-6. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

