HA-Coated PLGA Nanoparticles Loaded with Apigenin for Colon Cancer with High Expression of CD44
- PMID: 38005286
- PMCID: PMC10673172
- DOI: 10.3390/molecules28227565
HA-Coated PLGA Nanoparticles Loaded with Apigenin for Colon Cancer with High Expression of CD44
Abstract
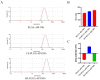
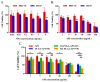
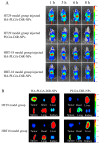
Apigenin (API) possesses excellent antitumor properties but its limited water solubility and low bioavailability restrict its therapeutic impact. Thus, a suitable delivery system is needed to overcome these limitations and improve the therapeutic efficiency. Poly (lactic-co-glycolic acid) (PLGA) is a copolymer extensively utilized in drug delivery. Hyaluronic acid (HA) is a major extracellular matrix component and can specifically bind to CD44 on colon cancer cells. Herein, we aimed to prepare receptor-selective HA-coated PLGA nanoparticles (HA-PLGA-API-NPs) for colon cancers with high expression of CD44; chitosan (CS) was introduced into the system as an intermediate, simultaneously binding HA and PLGA through electrostatic interaction to facilitate a tighter connection between them. API was encapsulated in PLGA to obtain PLGA-API-NPs, which were then sequentially coated with CS and HA to form HA-PLGA-API-NPs. HA-PLGA-API-NPs had a stronger sustained-release capability. The cellular uptake of HA-PLGA-API-NPs was enhanced in HT-29 cells with high expression of CD44. In vivo, HA-PLGA-API-NPs showed enhanced targeting specificity towards the HT-29 ectopic tumor model in nude mice in comparison with PLGA-API-NPs. Overall, HA-PLGA-API-NPs were an effective drug delivery platform for API in the treatment of colon cancers with high expression of CD44.
Keywords: API; CD44; HA; PLGA nanoparticles; colon cancer; targeted delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

