Hydroxy- and Hydro-Perfluoroalkylation of Styrenes by Controlling the Quenching Cycle of Eosin Y
- PMID: 38005299
- PMCID: PMC10674426
- DOI: 10.3390/molecules28227577
Hydroxy- and Hydro-Perfluoroalkylation of Styrenes by Controlling the Quenching Cycle of Eosin Y
Abstract
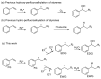
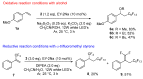
Fluoroalkyl compounds are widely used, underscoring a pressing need for the development of methods for their synthesis. However, reports on perfluoroalkylation to styrenes have been sparse. In this study, both hydroxy- and hydro-perfluoroalkylation of styrene were achieved using visible light reactions, catalyzed by eosin Y, by selecting appropriate additives and controlling the eosin Y quenching cycle. These reactions are heavy-metal free, use water as the hydroxyl or hydrogen source, and employ inexpensive and readily available reagents.
Keywords: eosin Y; perfluoroalkylation; photoredox catalyst; styrene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Metal-free visible-light-induced hydroxy-perfluoroalkylation of conjugated olefins using enamine catalyst.RSC Adv. 2022 Nov 15;12(51):32790-32795. doi: 10.1039/d2ra06679c. eCollection 2022 Nov 15. RSC Adv. 2022. PMID: 36425182 Free PMC article.
-
Visible-Light-Induced Metal-Free Decarboxylative Perfluoroalkylation of Aryl Acrylic Acids.Org Lett. 2022 Oct 21;24(41):7622-7626. doi: 10.1021/acs.orglett.2c03088. Epub 2022 Oct 11. Org Lett. 2022. PMID: 36219166
-
Photocatalytic/Cu-Promoted C-H Activations: Visible-light-Induced ortho-Selective Perfluoroalkylation of Benzamides.Chemistry. 2016 Apr 25;22(18):6218-22. doi: 10.1002/chem.201600229. Epub 2016 Mar 16. Chemistry. 2016. PMID: 26933840
-
Development of Electrophilic Radical Perfluoroalkylation of Electron-Deficient Olefins.Chem Rec. 2023 Sep;23(9):e202300037. doi: 10.1002/tcr.202300037. Epub 2023 Apr 14. Chem Rec. 2023. PMID: 37058111 Review.
-
Synthetic applications of eosin Y in photoredox catalysis.Chem Commun (Camb). 2014 Jun 28;50(51):6688-99. doi: 10.1039/c4cc00751d. Chem Commun (Camb). 2014. PMID: 24699920 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

