Galactooligosaccharide Mediates NF-κB Pathway to Improve Intestinal Barrier Function and Intestinal Microbiota
- PMID: 38005333
- PMCID: PMC10674247
- DOI: 10.3390/molecules28227611
Galactooligosaccharide Mediates NF-κB Pathway to Improve Intestinal Barrier Function and Intestinal Microbiota
Abstract
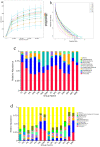
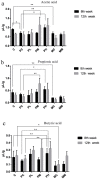
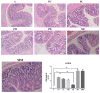
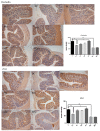
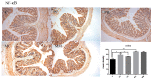
The use of antibiotics to treat diarrhea and other diseases early in life can lead to intestinal disorders in infants, which can cause a range of immune-related diseases. Intestinal microbiota diversity is closely related to dietary intake, with many oligosaccharides impacting intestinal microorganism structures and communities. Thus, oligosaccharide type and quantity are important for intestinal microbiota construction. Galactooligosaccharides (GOS) are functional oligosaccharides that can be supplemented with infant formula. Currently, information on GOS and its impact on intestinal microbiota diversity and disorders is lacking. Similarly, GOS is rarely reported within the context of intestinal barrier function. In this study, 16S rRNA sequencing, gas chromatography, and immunohistochemistry were used to investigate the effects of GOS on the intestinal microbiota and barrier pathways in antibiotic-treated mouse models. The results found that GOS promoted Bifidobacterium and Akkermansia proliferation, increased short-chain fatty acid levels, increased tight junction protein expression (occludin and ZO-1), increased secretory immunoglobulin A (SIgA) and albumin levels, significantly downregulated NF-κB expression, and reduced lipopolysaccharide (LPS), interleukin-IL-1β (IL-1β), and IL-6 levels. Also, a high GOS dose in ampicillin-supplemented animals provided resistance to intestinal damage.
Keywords: galactooligosaccharides; gut microbiota; intestinal barrier; short-chain fatty acids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Zhang L.Z., Gong J.G., Li J.H., Hao Y.S., Xu H.J., Liu Y.C., Feng Z.H. Dietary resveratrol supplementation on growth performance, immune function and intestinal barrier function in broilers challenged with lipopolysaccharide. Poultry Sci. 2023;102:102968. doi: 10.1016/j.psj.2023.102968. - DOI - PMC - PubMed
-
- Castro M., Valero M.S., López-Tofiño Y., López-Gómez L., Girón R., Martín-Fontelles M.I., Uranga J.A., Abalo R. Radiographic and histopathological study of gastrointestinal dysmotility in lipopolysaccharide-induced sepsis in the rat. Neurogastroent. Motil. 2023;35:e14639. doi: 10.1111/nmo.14639. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

