Optimizing the Composition of the Substrate Enhances the Performance of Peroxidase-like Nanozymes in Colorimetric Assays: A Case Study of Prussian Blue and 3,3'-Diaminobenzidine
- PMID: 38005344
- PMCID: PMC10674554
- DOI: 10.3390/molecules28227622
Optimizing the Composition of the Substrate Enhances the Performance of Peroxidase-like Nanozymes in Colorimetric Assays: A Case Study of Prussian Blue and 3,3'-Diaminobenzidine
Abstract
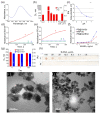
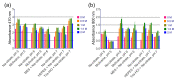
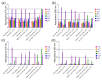
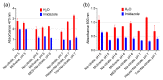
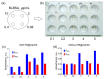
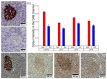
One of the emerging trends in modern analytical and bioanalytical chemistry involves the substitution of enzyme labels (such as horseradish peroxidase) with nanozymes (nanoparticles possessing enzyme-like catalytic activity). Since enzymes and nanozymes typically operate through different catalytic mechanisms, it is expected that optimal reaction conditions will also differ. The optimization of substrates for nanozymes usually focuses on determining the ideal pH and temperature. However, in some cases, even this step is overlooked, and commercial substrate formulations designed for enzymes are utilized. This paper demonstrates that not only the pH but also the composition of the substrate buffer, including the buffer species and additives, significantly impact the analytical signal generated by nanozymes. The presence of enhancers such as imidazole in commercial substrates diminishes the catalytic activity of nanozymes, which is demonstrated herein through the use of 3,3'-diaminobenzidine (DAB) and Prussian Blue as a model chromogenic substrate and nanozyme. Conversely, a simple modification to the substrate buffer greatly enhances the performance of nanozymes. Specifically, in this paper, it is demonstrated that buffers such as citrate, MES, HEPES, and TRIS, containing 1.5-2 M NaCl or NH4Cl, substantially increase DAB oxidation by Prussian Blue and yield a higher signal compared to commercial DAB formulations. The central message of this paper is that the optimization of substrate composition should be an integral step in the development of nanozyme-based assays. Herein, a step-by-step optimization of the DAB substrate composition for Prussian Blue nanozymes is presented. The optimized substrate outperforms commercial formulations in terms of efficiency. The effectiveness of the optimized DAB substrate is affirmed through its application in several commonly used immunostaining techniques, including tissue staining, Western blotting assays of immunoglobulins, and dot blot assays of antibodies against SARS-CoV-2.
Keywords: Prussian Blue; Western blotting; dot blot; immunoassay; immunohistochemistry; peroxidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Nanozymes: From New Concepts, Mechanisms, and Standards to Applications.Acc Chem Res. 2019 Aug 20;52(8):2190-2200. doi: 10.1021/acs.accounts.9b00140. Epub 2019 Jul 5. Acc Chem Res. 2019. PMID: 31276379
-
Protein-sized nanozymes «artificial peroxidase» based on template catalytic synthesis of Prussian Blue.Bioelectrochemistry. 2023 Feb;149:108275. doi: 10.1016/j.bioelechem.2022.108275. Epub 2022 Oct 1. Bioelectrochemistry. 2023. PMID: 36228395
-
Peroxidase-mimicking nanozyme with surface-dispersed Pt atoms for the colorimetric lateral flow immunoassay of C-reactive protein.Mikrochim Acta. 2021 Aug 27;188(9):309. doi: 10.1007/s00604-021-04968-x. Mikrochim Acta. 2021. PMID: 34453188
-
Prussian blue: from advanced electrocatalyst to nanozymes defeating natural enzyme.Mikrochim Acta. 2022 Jul 25;189(8):290. doi: 10.1007/s00604-022-05363-w. Mikrochim Acta. 2022. PMID: 35879483 Review.
-
Recent advances in colorimetric sensors based on nanozymes with peroxidase-like activity.Analyst. 2023 Jan 31;148(3):487-506. doi: 10.1039/d2an01850k. Analyst. 2023. PMID: 36484756 Review.
Cited by
-
Pt@ZnCo2O4 Microspheres as Peroxidase Mimics: Enhanced Catalytic Activity and Application for L-Cysteine Detection.Molecules. 2025 Jan 5;30(1):187. doi: 10.3390/molecules30010187. Molecules. 2025. PMID: 39795244 Free PMC article.
References
-
- Wang Q., Wei H., Zhang Z., Wang E., Dong S. Nanozyme: An Emerging Alternative to Natural Enzyme for Biosensing and Immunoassay. TrAC Trends Anal. Chem. 2018;105:218–224. doi: 10.1016/j.trac.2018.05.012. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

